Method for generating glycol through two-step catalytic hydrogenation reaction of oxalate
A technology for catalytic hydrogenation and oxalate ester, applied in chemical instruments and methods, production of bulk chemicals, preparation of organic compounds, etc. The effect of magnifying the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
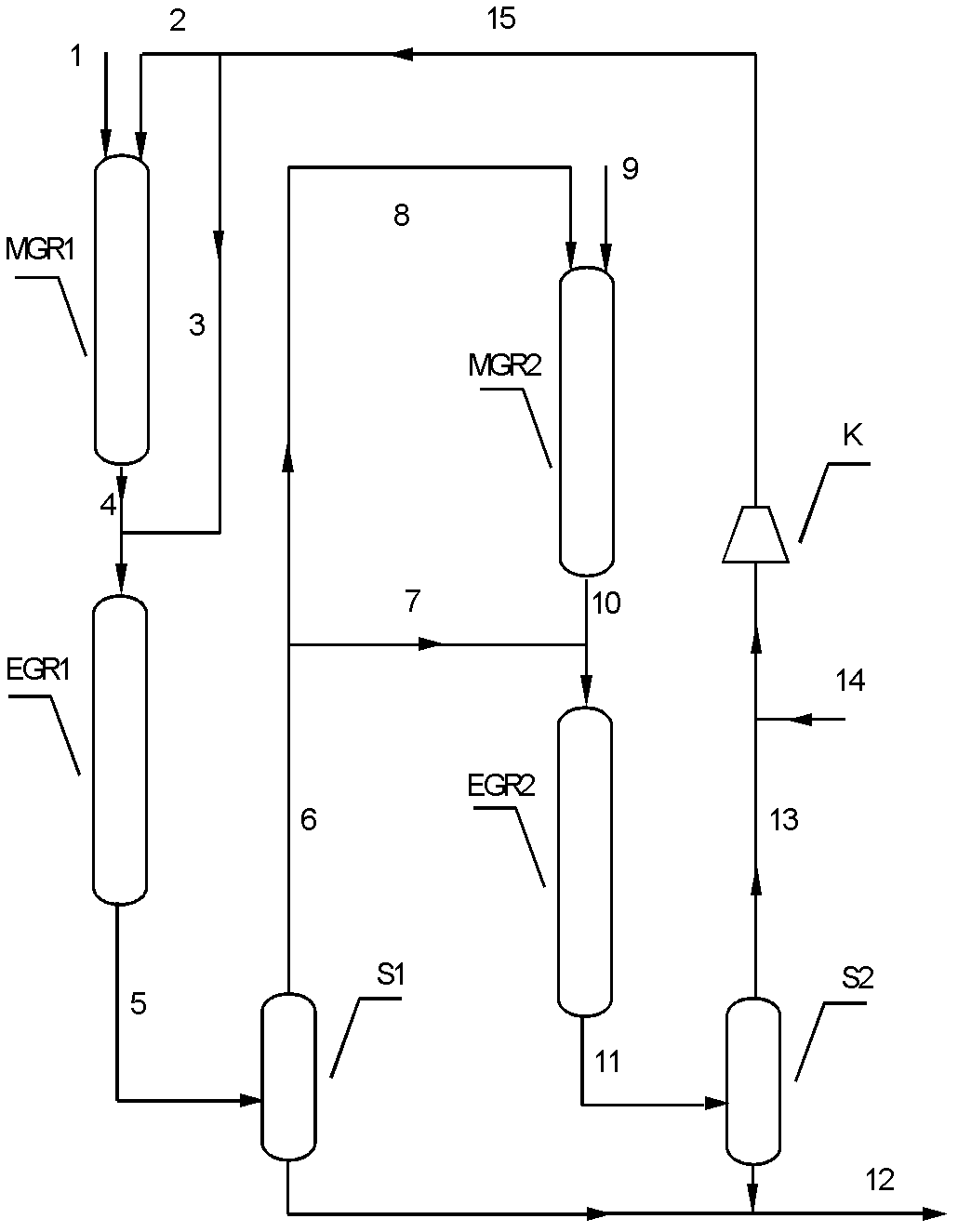
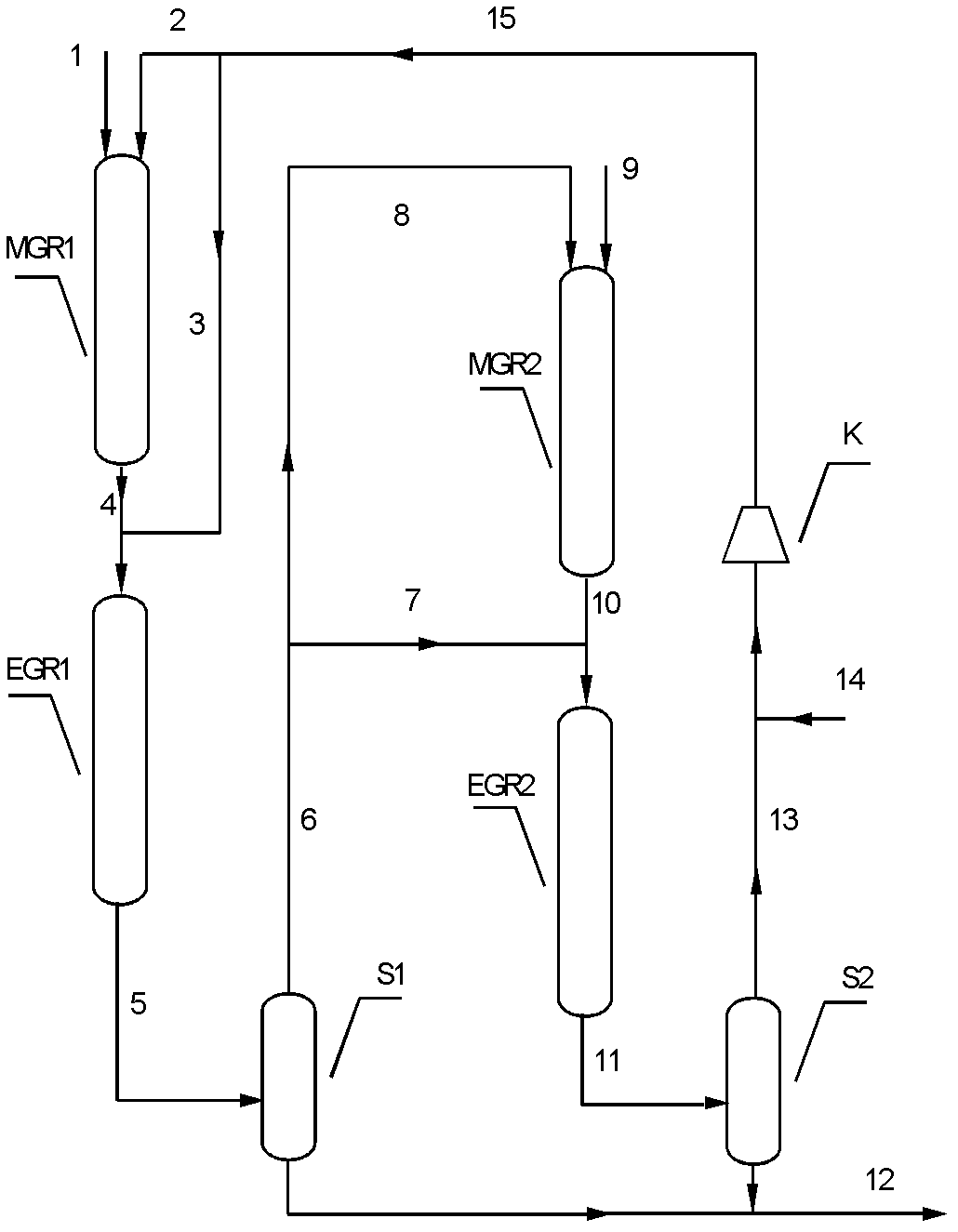
[0032] Such as figure 1 Shown, adopt technological process of the present invention to test.
[0033] First prepare Cu-Zn / SiO 2 The catalyst, wherein the weight content of Cu is 20%, and the weight content of Zn is 5%. Cu with (Cu+CuO+Cu 2 O) form exists in the catalyst, and Zn exists in the catalyst in the form of (Zn+ZnO). Catalyst preparation method is as follows: first weigh specific surface area 215m 2 / g of 200g of silica carrier, weigh a certain amount of copper nitrate and zinc nitrate to prepare a solution, impregnate and stir the silica carrier in the solution for 24 hours, vacuum dry at room temperature for 12 hours, and then dry at 120C 24 hours, followed by calcination at 400 °C for 6 hours to prepare the catalyst precursor. Before use, it was reduced with pure hydrogen at a volumetric space velocity of 2000 / h, raised from room temperature to 350 °C at a rate of 2 °C / min, and reduced at normal pressure for 8 hours to obtain an active catalyst.
[0034] Secon...
Embodiment 2
[0047] Adopt the Cu-Zn / SiO that embodiment 1 is used simultaneously 2 Catalysts and Cu-Co / SiO 2 catalyst.
[0048] According to attached figure 1 The process flow shown is that two glycolate synthesis reactors are filled with the same weight of Cu-Zn / SiO 2 Catalyst, the same weight of Cu-Co / SiO was loaded in two ethylene glycol synthesis reactors 2 catalyst. The feed was dimethyl oxalate and hydrogen with a purity of 99.8%. The reaction temperature in the first glycolate synthesis reactor is 180°C, the reaction pressure is 3.0MPa, the reaction weight space velocity is 1.5 / h, and the molar ratio of hydrogen to dimethyl oxalate is 40:1; the first ethylene glycol synthesis reactor The reaction temperature is 210° C., the reaction pressure is 2.8 MPa, the reaction weight space velocity is 1.5 / h, and the molar ratio of hydrogen to glycolate is 60:1. In the second glycolate synthesis reactor, the reaction temperature is 180°C, the reaction pressure is 2.6MPa, the reaction weig...
Embodiment 3
[0060] Adopt the Cu-Zn / SiO that embodiment 1 is used simultaneously 2 Catalysts and Cu-Co / SiO 2 catalyst.
[0061] According to the process flow shown in the accompanying drawing, the same weight of Cu-Zn / SiO is filled in two glycolate synthesis reactors 2 Catalyst, the same weight of Cu-Co / SiO was loaded in two ethylene glycol synthesis reactors 2 catalyst. The feed was dimethyl oxalate and hydrogen with a purity of 99.8%. The reaction temperature in the first glycolate synthesis reactor is 160°C, the reaction pressure is 3.0MPa, the reaction weight space velocity is 4.0 / h, and the molar ratio of hydrogen to dimethyl oxalate is 80:1; the first ethylene glycol synthesis reactor The reaction temperature is 230° C., the reaction pressure is 2.8 MPa, the reaction weight space velocity is 4.0 / h, and the molar ratio of hydrogen to glycolate is 120:1. In the second glycolate synthesis reactor, the reaction temperature is 160°C, the reaction pressure is 2.6MPa, the reaction weig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com