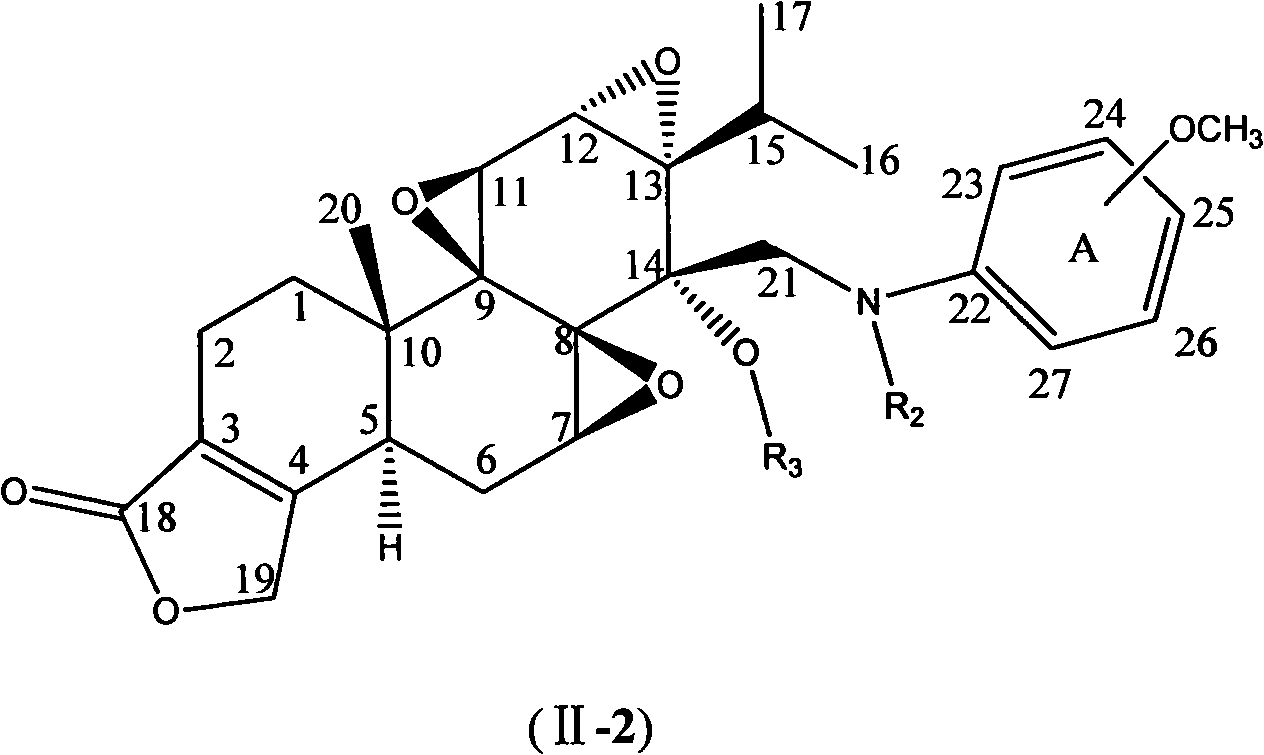
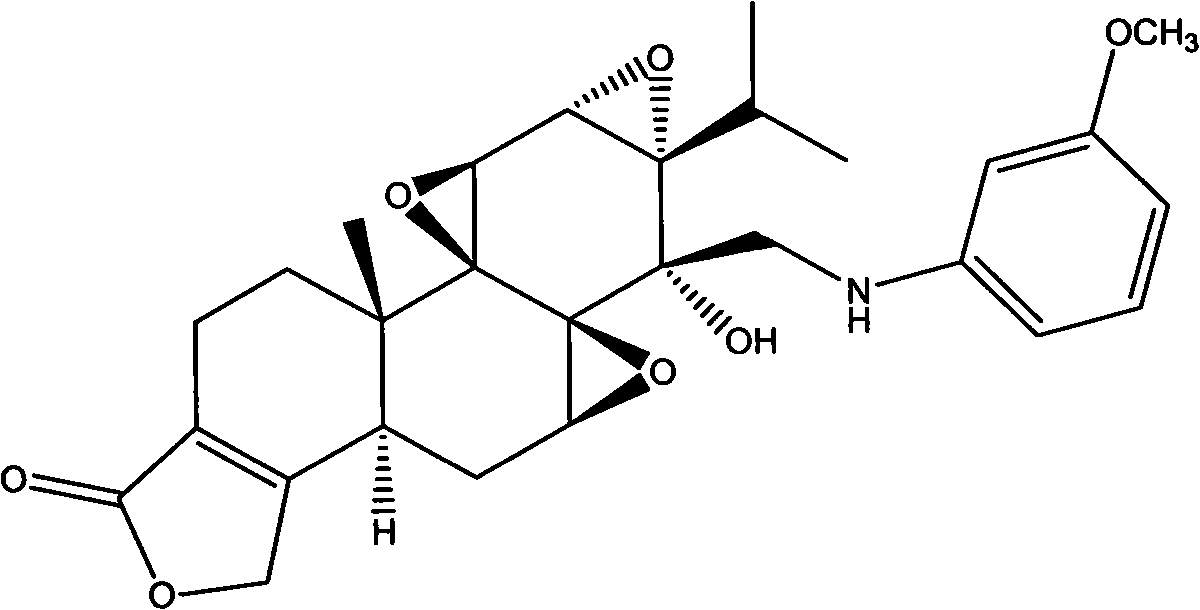
N-substituted methoxy-containing phenyl-14 Beta-aminomethyl epitriptolide derivatives, preparation method and use thereof
A technology of methoxyphenyl and aminomethyl forms, applied in the field of medicine, can solve the problems of large toxic side effects, narrow treatment window, and no fundamental changes in the main structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0055] Preparation Example 1 compound (3)
[0056]
[0057] Under argon protection, 50 mL of anhydrous tetrahydrofuran was added into a reaction flask containing magnesium chips (900 mg, 37.5 mmol), and chloromethyldimethylisopropoxysilane (6 mL) was added dropwise to the reaction flask via a constant pressure dropping funnel. in the reaction system. After the addition, the dark gray reaction system was stirred at 50° C. for 30 min, and the Grignard reagent was prepared. The prepared Grignard reagent was added dropwise to triptolide ketone (LLDT-1) (3 g, 8.4 mmol) dissolved in 100 mL of dry THF. After reacting for 1.5 h at room temperature, the reaction was stopped. The reaction system was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain the crude compound (2). Without further purification, compound (2) was dissolved in 50 mL ...
preparation Embodiment 2
[0059] Preparation Example 2 Compound (4)
[0060]
[0061] Compound 3 (420mg, 1.08mmol) was dissolved in 15mL of dichloromethane solvent, trichloroisocyanuric acid (376mg, 1.62mmol) was added at 0°C, followed by TEMPO (16mg, 0.108mmol) and quickly detected by TLC, the reaction Completely add sodium carbonate solution to quench the reaction and adjust the pH value to neutral, extract with dichloromethane, wash the organic phase with water and saturated brine respectively, dry over anhydrous sodium sulfate and concentrate, separate and purify by column chromatography to obtain compound 4 as a white solid (340 mg, 0.87 mmol, yield: 81%).
[0062] Compound 4: 1 H NMR (CDCl 3 , 300MHz) δ10.03(s, 1H), 4.76-4.59(m, 2H), 3.97(d, J=3.0Hz, 1H), 3.91(s, 1H), 3.75(d, J=5.9Hz, 1H ), 3.60(d, J=3.0Hz, 1H), 2.79-2.67(m, 1H), 2.38-2.26(m, 1H), 1.87(dd, J=14.7, 13.6Hz, 1H), 1.58(dd, J=12.6, 4.0Hz, 1H), 1.03(s, 3H), 0.83(d, J=6.9Hz, 3H), 0.79(d, J=6.9Hz, 3H); 13 C NMR (CDCl 3 , 100MHz)...
preparation Embodiment 3
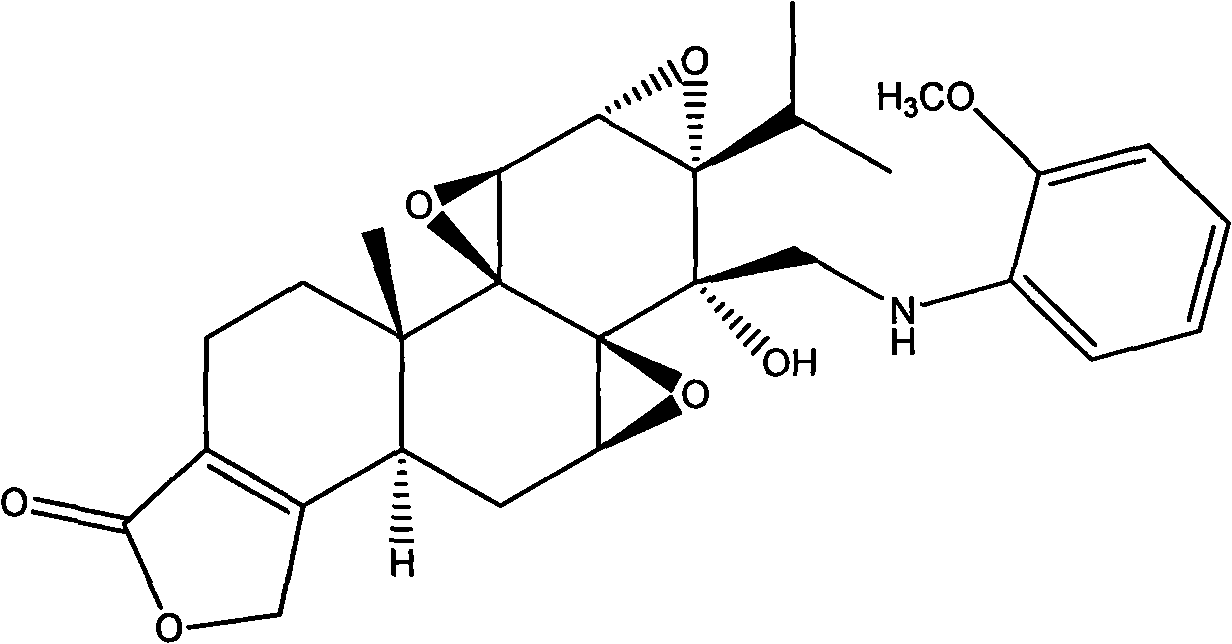
[0063] Preparation Example 3 (14S)-14β-N-(2′-methoxyphenyl)-aminomethyl epitriptolide (LLDT-208)
[0064]
[0065] Compound 4 (39 mg, 0.1 mmol) was dissolved in 4 mL of acetonitrile solvent, o-methoxyaniline (12.3 mg, 0.1 mmol) was added, stirred at room temperature for 0.5 h, and then sodium triacetoxyborohydride (42 mg, 0.2 mmol) was added , after reacting at room temperature for 4 hours, the reaction was stopped, most of the solvent was evaporated under reduced pressure, the residue was diluted with water and extracted with ethyl acetate, the organic phase was washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, concentrated, and the column layer Analysis, separation and purification gave white solid compound (14S)-14β-N-(2′-methoxyphenyl)-aminomethyl epitriptolide (LLDT-208) (34.6 mg, yield: 70%).
[0066] LLDT-208: 1 H NMR (CDCl 3 , 300MHz) δ6.92-6.73(m, 4H), 4.75-4.62(m, 3H), 3.90-3.80(m, 7H), 3.54-3.45(m, 2H), 2.72(m, 1H), 2.51( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com