Method for inducing cell death in pluripotent stem cells and differentiated cells other than cardiac myocytes
一种多能性干细胞、心肌细胞的技术,应用在对多能性干细胞和心肌细胞以外的分化细胞诱导细胞死亡领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Embryonic stem cell-derived cardiomyocytes and remaining undifferentiated embryos Immunostaining of Stem Cells
[0075] The purpose of this example is to confirm the mixed state of cardiomyocytes and undifferentiated cells in the cell mass (embryoid body) when the cell mass (embryoid body) containing cardiomyocytes was formed from stem cells.
[0076] Mouse embryonic stem cells (cell line name EB3, Nat Genet 2000; 24: 372-376) were kindly donated by Dr. Hitoshi Niwa of RIKEN. For the mouse embryonic stem cells, use the existing method (Bader A, et al., Differentiation 2001, 68, p31-43) (that is, use culture medium [α-MEM (Minimum Essential Medium, minimum essential medium) ( SIGMA), 10% FBS (EQUITEC BIO), 100 units / ml penicillin, 50 μg / ml streptomycin (GIBCO)] By the hanging drop method, 75 embryonic stem cells were cultured in the form of cell block for each EB for 7 days. Method) After differentiating the cell mass containing cardiomyocytes, the embryo...
Embodiment 2
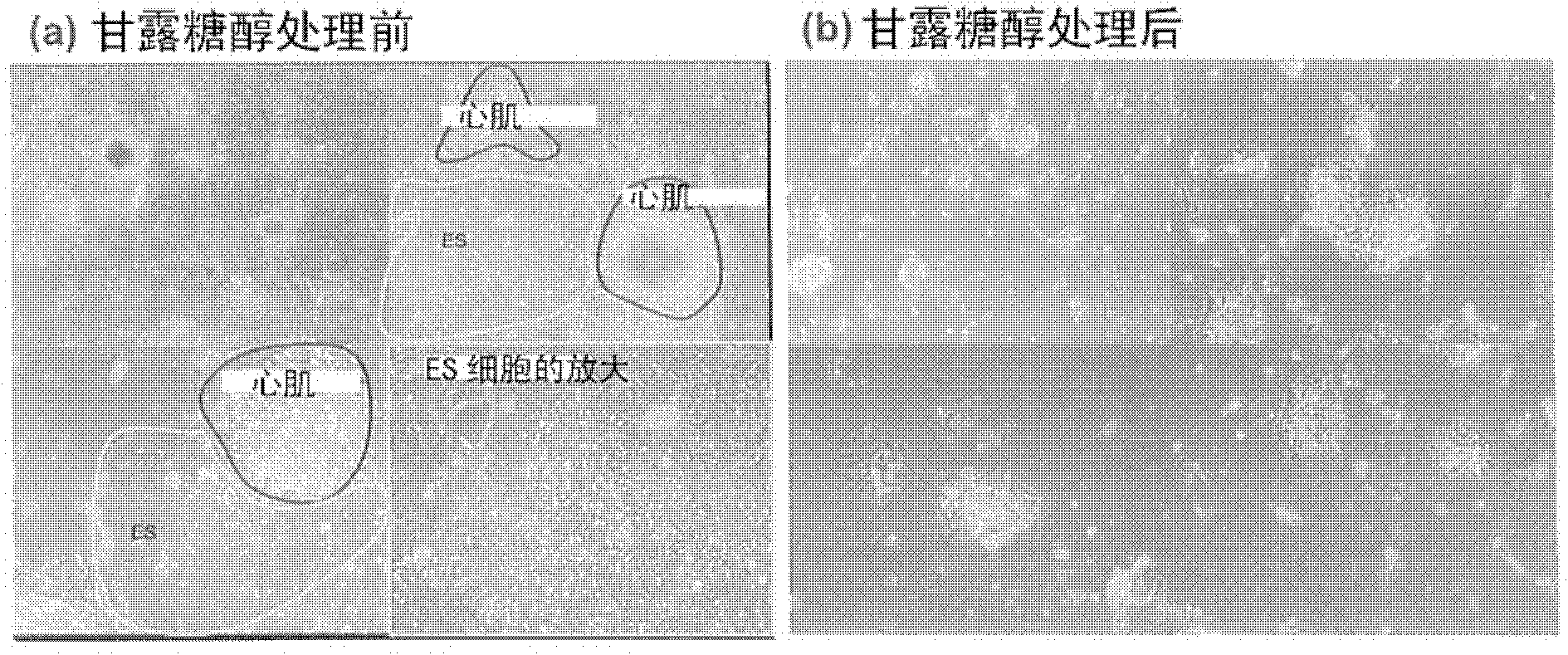
[0079] Example 2: After mixing cardiomyocytes derived from embryonic stem cells and remaining embryos Sugar (sugar alcohols) treatment in the culture system of fetal stem cells
[0080] The purpose of this example is to confirm the culture state of cardiomyocytes and the culture state of other cells after treating a cell mass (embryoid body) containing cardiomyocytes formed from embryonic stem cells with sugar (sugar alcohols).
[0081] Mouse-derived embryonic stem cells were formed into embryoid bodies by the method described in Example 1 and differentiated to a stage containing programmed cardiomyocytes (precardiac mesoderm). The resulting embryoid bodies were carefully treated with digestive enzymes (trypsin, collagenase) to prevent cell damage, thereby partially dispersing them, and culturing them again for adherence. When continuing to culture for 5 days, if figure 2 As shown, a group of cardiomyocytes beating autonomously (the cell group surrounded by the red line)...
Embodiment 3
[0084] Embodiment 3: Glucose (sugar alcohols) induces cell death of mouse embryonic stem cells Guidance
[0085] The purpose of this example is to confirm the survival state of stem cells after treating mouse embryonic stem cells with sugars (sugar alcohols).
[0086] As for the mitochondrial membrane potential, which is lost in dead cells, when the membrane potential as an indicator of survival is detected and stained with the fluorescent mitochondrial membrane potential sensitive reagent TMRM (Molecular Probes), the reagent-derived The fluorescent signal is higher in live cells and becomes lower in dead cells.
[0087] Utilizing this property, the embryonic stem cells cultured by the method described in Example 1 were cultured in α-MEM medium containing mannitol 0.45M (equivalent to about 720mOsm / kg) and 1 μM TMRM for 0h (initial), 3h , 6h, and 20h later, the collected embryonic stem cells were washed, and then analyzed by FACS. Based on the fluorescence intensity of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com