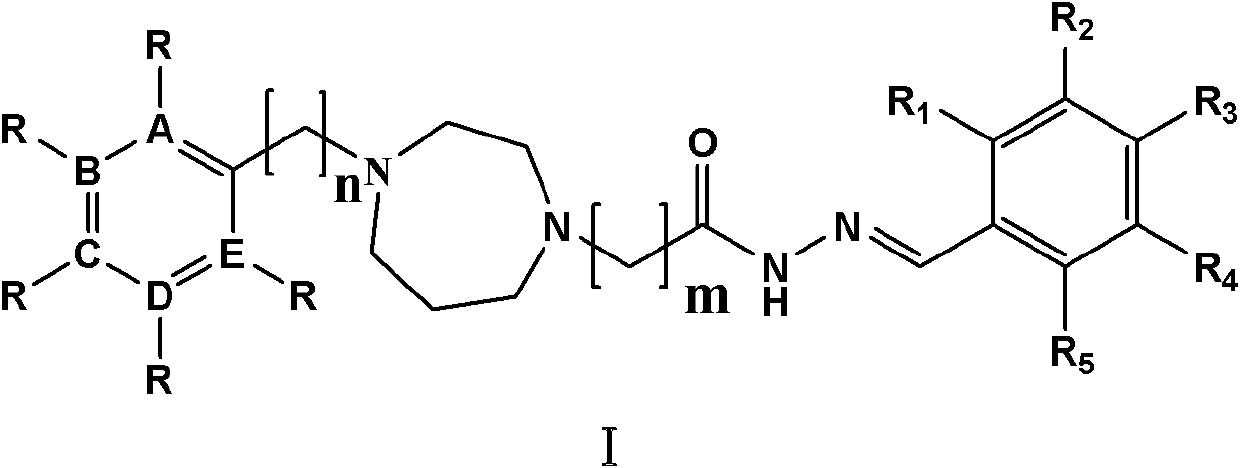
Homopiperazine acethydrazide derivative with low neurotoxicity and preparation method and application thereof
A compound, prodrug technology, applied in the field of preparing said compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
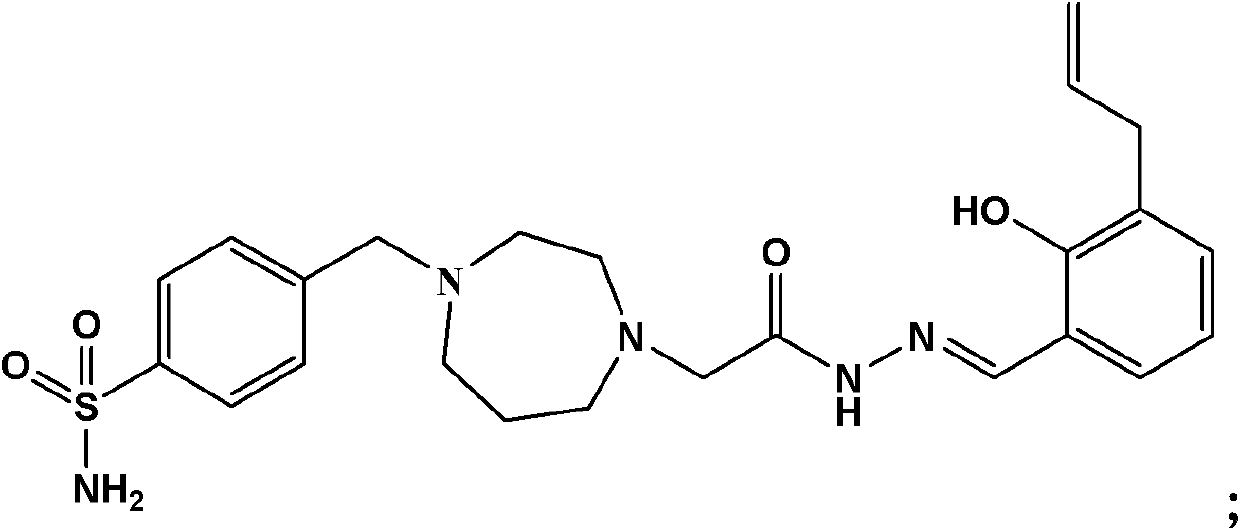
[0130] Example 1, 2-(4-p-sulfonamidobenzyl-[1,4]diazepan-1-yl)-acetyl(3-allyl-2-hydroxyl-methylenebenzene) Preparation of hydrazine fumaric acid
[0131] The reaction process is as follows:
[0132]
[0133] Step 1. At room temperature, under the condition of electromagnetic stirring, a mixed solution of 2.45g (20mmol) ethyl 2-chloroacetate and 20ml methanol was added dropwise in a reaction flask equipped with 10g (100mmol) homopiperazine and 20ml methanol. The dropwise addition was completed within half an hour, stirred at room temperature for 5 hours, after the reaction was completed, filtered, the filter cake was washed with a small amount of acetone, the filtrate was combined, concentrated to remove methanol, water was added to the raffinate, extracted twice with ethyl acetate, combined, anhydrous MgS0 4 After drying, concentration, and silica gel column separation of the residue, 1.86 g of the product ([1,4]diazepan-1-yl)ethyl acetate was obtained, with a yield of 50...
Embodiment 2
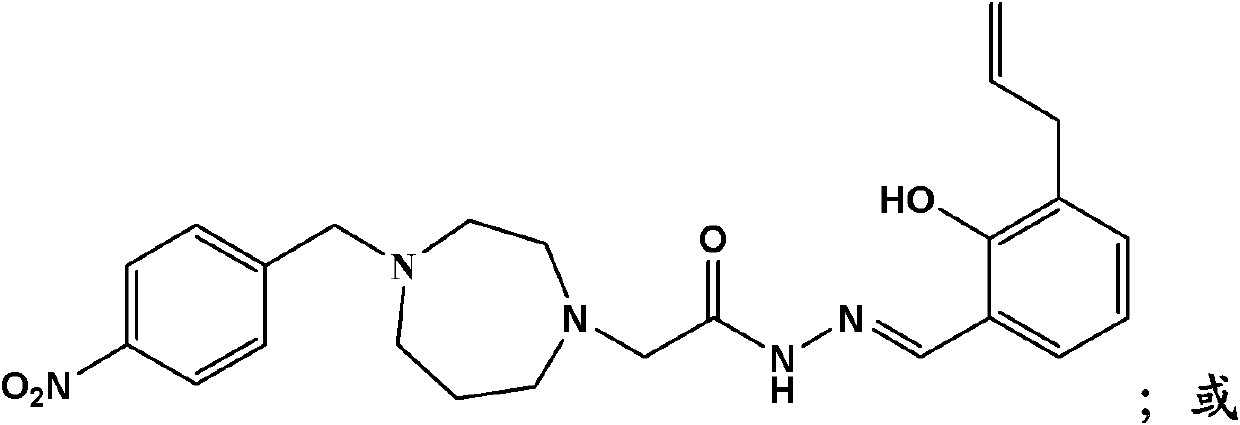
[0138] Example 2, 2-(4-p-nitrobenzyl-[1,4]diazepan-1-yl)-acetyl(3-allyl-2-hydroxyl-methylenephenyl)hydrazine preparation of
[0139] The reaction process is as follows:
[0140]
[0141] Step 1. At room temperature, under the condition of electromagnetic stirring, add ([1,4]diazepan-1-yl) ethyl acetate and 1.01g ( 10mmol) of triethylamine, stirred evenly, then added 2.16g (10mmol) of p-nitrobenzyl bromide in batches, reacted at room temperature for 6 hours, and stopped the reaction after the end of the detection reaction. Filter, wash the filter cake with a small amount of acetone, combine the filtrates, concentrate, and purify the residue on a silica gel column to obtain off-white solid product (4-p-nitrobenzyl-[1,4]diazepan-1-yl)acetic acid Ethyl ester 3g, yield 93.4%. Mass spectrum: 322 (M+1).
[0142] Step 2. At room temperature, under the condition of electromagnetic stirring, add 5ml of methanol and 1.61g (5mmol) of (4-p-nitrobenzyl-[1,4]diazepan-1-yl to the react...
Embodiment 3
[0144] Example 3, 2-(4-m-carboxybenzyl-[1,4]diazepan-1-yl)-acetyl (3-allyl-2-hydroxyl-methylenephenyl)hydrazine preparation
[0145] The reaction process is as follows:
[0146]
[0147]Step 1. At room temperature, under the condition of electromagnetic stirring, add 10ml acetone, 1.86g (10mmol) ([1,4]diazepan-1-yl) ethyl acetate and 2.02g ( 20mmol) of triethylamine, stirred evenly, then added 1.70g (10mmol) of m-chloromethylbenzoic acid in batches, reacted at room temperature for 6 hours, and stopped the reaction after the detection reaction was completed. Filtration, the filter cake was washed with a small amount of acetone, the combined filtrate was concentrated, and the residue was purified by a silica gel column to obtain an off-white solid product (4-m-carboxybenzyl-[1,4]diazepan-1-yl) ethyl acetate Esters 2.4g, yield 75%. Mass spectrum: 321 (M+1).
[0148] Step 2. At room temperature, under the condition of electromagnetic stirring, add 5ml of methanol and 1.61g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com