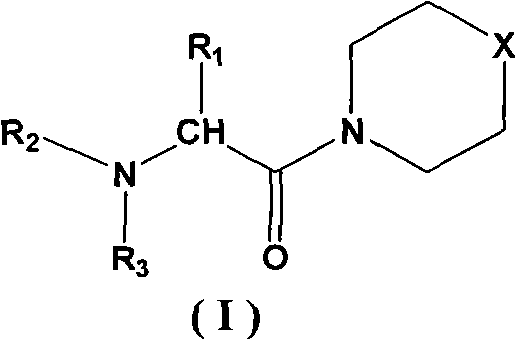
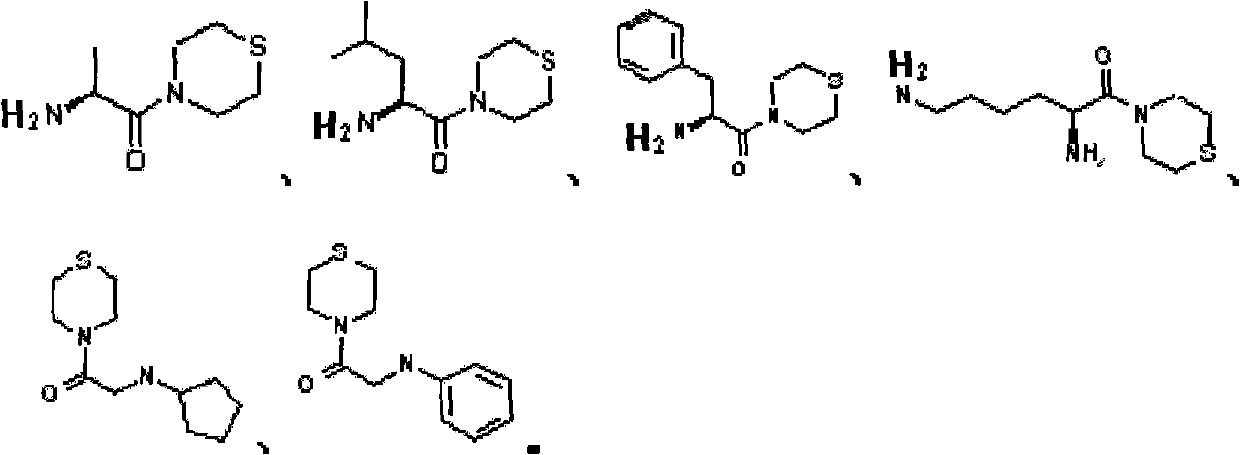
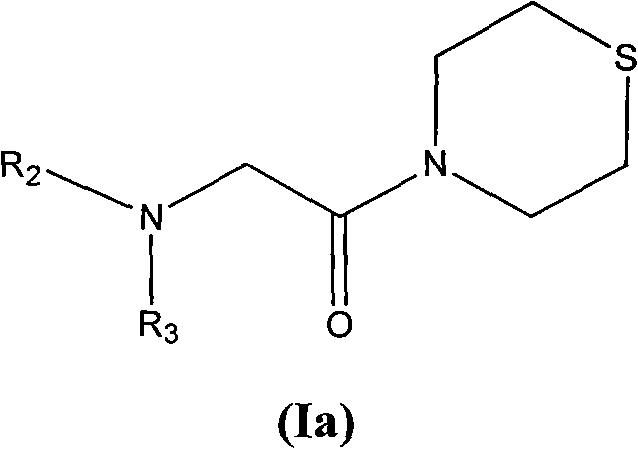
Sulfomorpholine compounds, preparation method thereof and purpose thereof
A compound and solvate technology, applied in the field of medicine, can solve problems such as research and development difficulties, few drugs on the market, and inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0219] Preparation Route 1. Preparation of BOC-Amino Acids
[0220]
[0221] Starting materials: various natural L-amino acids.
[0222] Reaction conditions: the reaction is preferably carried out under alkaline conditions, the preferred alkali is alkali metal hydroxide or aluminum oxide, and the preferred alkali metal hydroxide is potassium hydroxide, sodium hydroxide.
[0223] Reaction solvent: 1,4-dioxane is used as a solvent, and other solvents, such as water, are selectively added according to the solubility of different amino acid derivatives and the base used. The amount of the solvent is appropriately adjusted according to the solubility of the reactants.
[0224] Temperature: The reaction temperature is preferably 5-40°C, more preferably 10-30°C, most preferably 15-25°C.
[0225] Time: The reaction time is 1-40 hours, preferably 5-30 hours, more preferably 14-25 hours.
[0226] The progress of the reaction can be monitored by thin layer chromatography (TLC) or...
specific Embodiment approach
[0309] The present invention can be further described by the following examples, however, the scope of the present invention is not limited to the following examples. Those skilled in the art can understand that various changes and modifications can be made in the present invention without departing from the spirit and scope of the present invention. The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible.
[0310] For all of the following examples, standard manipulations and purification methods known to those skilled in the art can be used. All temperatures are in °C (degrees Celsius) unless otherwise indicated. Compound structures were determined by nuclear magnetic resonance (NMR) or mass spectroscopy (MS)....
Embodiment 1
[0313]
[0314] 4-(2-aminoacetyl)thiomorpholine hydrochloride compound 1
[0315] Step 1: N-tert-butoxycarbonylglycine
[0316] Glycine (1.5g, 20mmol) was dissolved in 20ml of 2M NaOH, and added at 0°C (BOC) 2 O (5.24g, 24mmol), raised to room temperature and reacted, the reaction was complete after 4 hours, the reaction solution was adjusted to pH=2-3 with concentrated hydrochloric acid, extracted with ethyl acetate (50ml*3), dried over anhydrous sodium sulfate , filtered, and concentrated to dryness under reduced pressure to obtain 2.1 g of white solid, yield: 60%.
[0317] Step 2: 4-[2-(N-tert-butoxycarbonylamino)-acetyl]thiomorpholine
[0318] Dissolve N-tert-butoxycarbonylglycine (0.18g, 1mmol) in 10ml of dichloromethane, add EDC (0.23g, 1.2mmol), stir well, add thiomorpholine (0.12g, 1.2mmol), and stir at room temperature . After 3 hours, the reaction was complete, and the reaction solution was passed through a vacuum column (silica gel H, petroleum ether: ethyl a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com