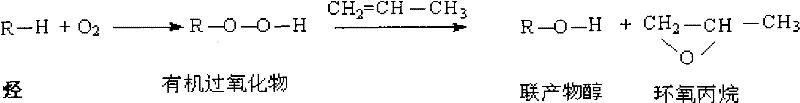Method for preparing epoxypropane
A technology of propylene oxide and epoxidation, which is applied in the direction of organic chemistry, can solve the problems of no breakthrough progress, and achieve the effects of high production efficiency, high reaction selectivity, and high peroxide concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
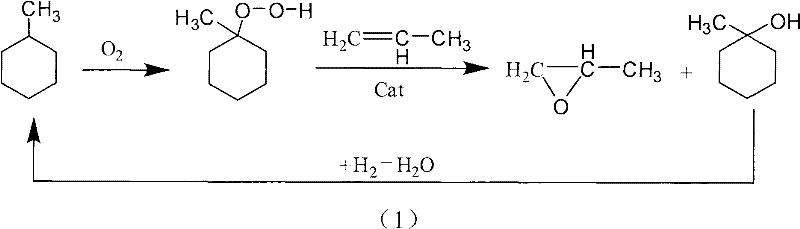
Embodiment 1
[0079] The first step, the oxidation reaction:
[0080]In a 500ml stainless steel reaction kettle equipped with a stirring and condensing reflux device, add 200ml of methylcyclohexane as the reaction raw material, seal the reaction kettle, heat up to and keep the temperature at the reaction temperature of 135°C under a nitrogen atmosphere, and adjust the reaction pressure to a gauge pressure of 0.4MPa , oxidize by feeding air at a speed of 400ml / min, and stir at a speed of 500rmp. When the reaction time was 10 h, the collected reaction solution was used as the raw material for the second step reaction.
[0081] During the experiment, the liquid material samples in the reactor were taken through the sampling port every 1 h, and the concentration of methylcyclohexyl hydroperoxide in the samples was analyzed by traditional iodometric method. At the initial stage of the reaction, the concentration of methylcyclohexyl hydroperoxide increases with the prolongation of the reaction t...
Embodiment 2
[0086] Embodiment 2: the first step oxidation reaction
[0087] Add 200ml of methylcyclohexane to a 500ml stainless steel reaction kettle equipped with a stirring and condensing reflux device, seal it, heat up to and keep the temperature at a reaction temperature of 100°C under a nitrogen atmosphere, adjust the reaction pressure to a gauge pressure of 0.3MPa, and use 400ml / The gas with an oxygen content of 30%, that is, oxygen-enriched air, is passed through at a speed of min for oxidation, and the stirring speed is 500rmp. When the reaction time was 12 hours, the concentration of methylcyclohexyl hydroperoxide in the reaction liquid reached the highest, which was 12.5%. Collect the reaction liquid as the raw material of the second step epoxidation reaction.
Embodiment 3
[0088] Embodiment 3: the first step oxidation reaction
[0089] Add 200ml of methylcyclohexane to a 500ml stainless steel reaction kettle equipped with a stirring and condensing reflux device, seal it, heat up to and keep the temperature at a reaction temperature of 145°C under a nitrogen atmosphere, adjust the reaction pressure to a gauge pressure of 1.0MPa, and use 400ml / The gas with an oxygen content of 15%, that is, oxygen-depleted air, is passed through at a speed of min for oxidation, and the stirring speed is 500rmp. When the reaction time was 8 hours, the concentration of methylcyclohexyl hydroperoxide in the reaction liquid reached the highest, which was 22.4%. Collect the reaction liquid as the raw material of the second step epoxidation reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com