Oxazolidinone derivative and preparation method and application thereof
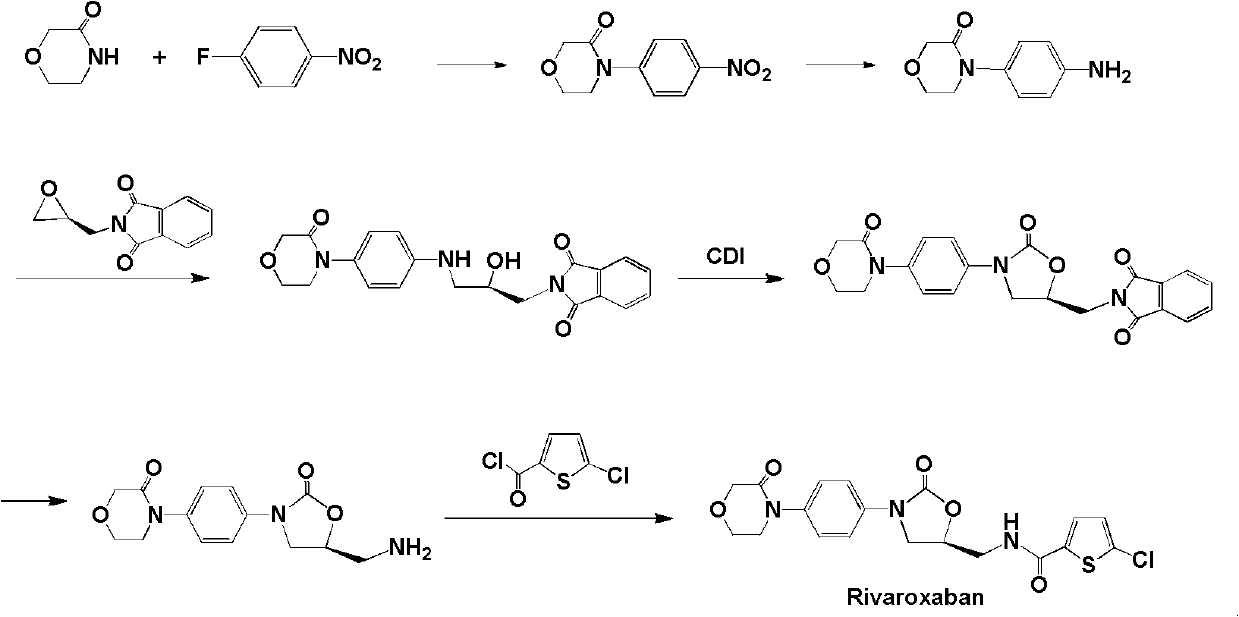
A technology of oxazolidine and medicine, which is applied in the field of blood coagulation, can solve the problems such as difficult purification of rivaroxaban, and achieve the effect of low price, easy preparation and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
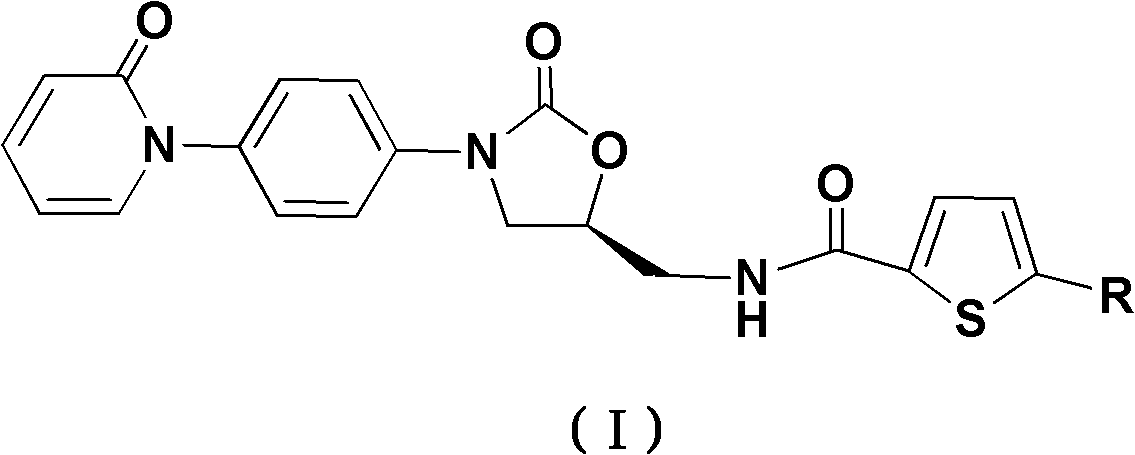
[0046] (S)-5-Chloro-N-((2-oxo-3-(4-(2-oxo-2H-pyridin-1-yl)phenyl)-1,3-oxazolidine-5- (Yl)methyl)thiophene-2-carboxamide (I-1)
[0047]
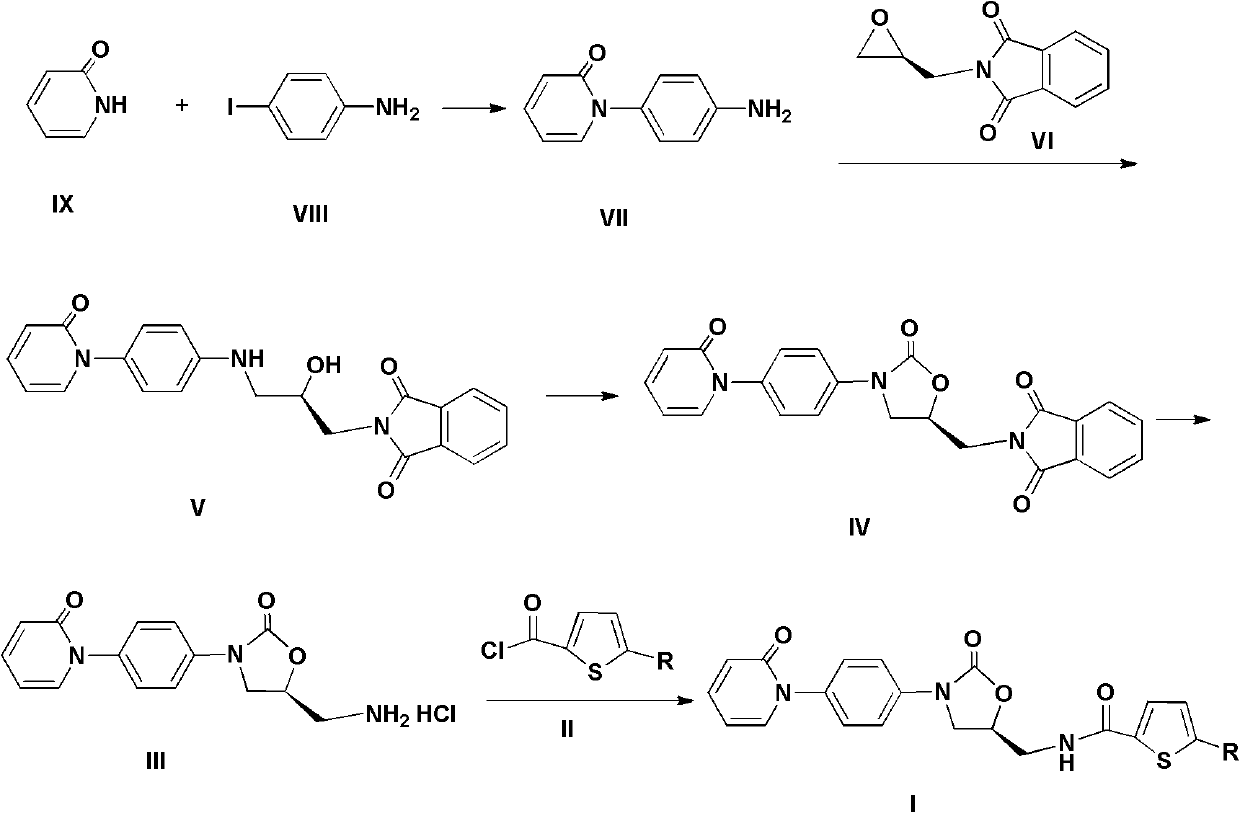
[0048] A. Synthesis of 1-(4-aminophenyl)-1H-pyridin-2-one (Compound VII)
[0049] Add 104g pyridine-2(1H)-one (compound IX), 200g p-iodoaniline (compound VIII), 26gCuI, 151g potassium carbonate, 18g8-hydroxyquinoline, 500ml DMF, nitrogen protection, heating and refluxing, heat preservation and stirring to the reaction flask. 10h. Filter while hot, take the filtrate, evaporate the solvent under reduced pressure, add 1L ethyl acetate to the residue, keep stirring at 0°C for 1h, filter, dry the solid, and refine with 2L acetonitrile to obtain 98g dark red solid. The refined mother liquor was concentrated to 500ml, stirred in an ice bath for 1h, and filtered to obtain 19g of dark red solid. A total of 117 g of product was obtained, with a yield of 68.9%.
[0050] 1 H-NMR(DMSO-d 6 ), δ (ppm): 5.306 (s, 2H), 6.236 (d, 1H), 6.406 (d, 1H), 6.601 (d, 2H)...
Embodiment 2
[0074] (S)-5-Bromo-N-((2-oxo-3-(4-(2-oxo-2H-pyridin-1-yl)phenyl)-1,3-oxazolidine-5- (Yl)methyl)thiophene-2-carboxamide (I-2)
[0075]
[0076] Melting point: 200.8~201.8℃;
[0077] 1 H-NMR(DMSO-d 6 ), δ (ppm): 3.580 (t, 2H), 3.902 (m, 1H), 4.221 (t, 1H), 4.849 (m, 1H), 6.308 (t, 1H), 6.468 (d, 1H), 7.193 (d, 1H), 7.426 (m, 2H), 7.500 (m, 1H), 7.637 (m, 4H), 8.967 (t, 1H);
[0078] MS(ESI): m / z=474(M+H);
[0079] HPLC: rt(%)=13.44(99.55);
[0080] [α] 20 D = -32.9° (c 0.3010, DMSO);
Embodiment 3
[0082] (S)-5-methyl-N-((2-oxo-3-(4-(2-oxo-2H-pyridin-1-yl)phenyl)-1,3-oxazolidine-5 -Yl)methyl)thiophene-2-carboxamide (I-3)
[0083]
[0084] 1 H-NMR(DMSO-d 6 ), δ (ppm): 3.457 (s, 3H), 3.600 (t, 2H), 3.897 (m, 1H), 4.224 (t, 1H), 4.860 (m, 1H), 6.314 (t, 1H), 6.468 (d, 1H), 6.842 (d, 1H), 7.419 (d, 2H), 7.499 (m, 1H), 7.622 (m, 4H), 8.724 (t, 1H).
[0085] MS(ESI): m / z=410(M+H);
[0086] In the HPLC data given in the above examples, the unit of retention time rt is minutes, and the HPLC parameters are:
[0087] Instrument: Waters 996-717-600 liquid chromatograph; Column: GL Science C18 column; Column temperature: 45°C; Eluent: Phase A is potassium dihydrogen phosphate solution, Phase B is potassium dihydrogen phosphate solution-acetonitrile ( 20:80); detection wavelength: 233nm; flow rate: 1.2ml / ml; gradient: 0.0min 55%A→5min 55%A→10min 40%A→20min 0%A→35min 0%A→36min 55%A.
[0088] In order to more fully explain the implementation of the present invention, the following pharmacolog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com