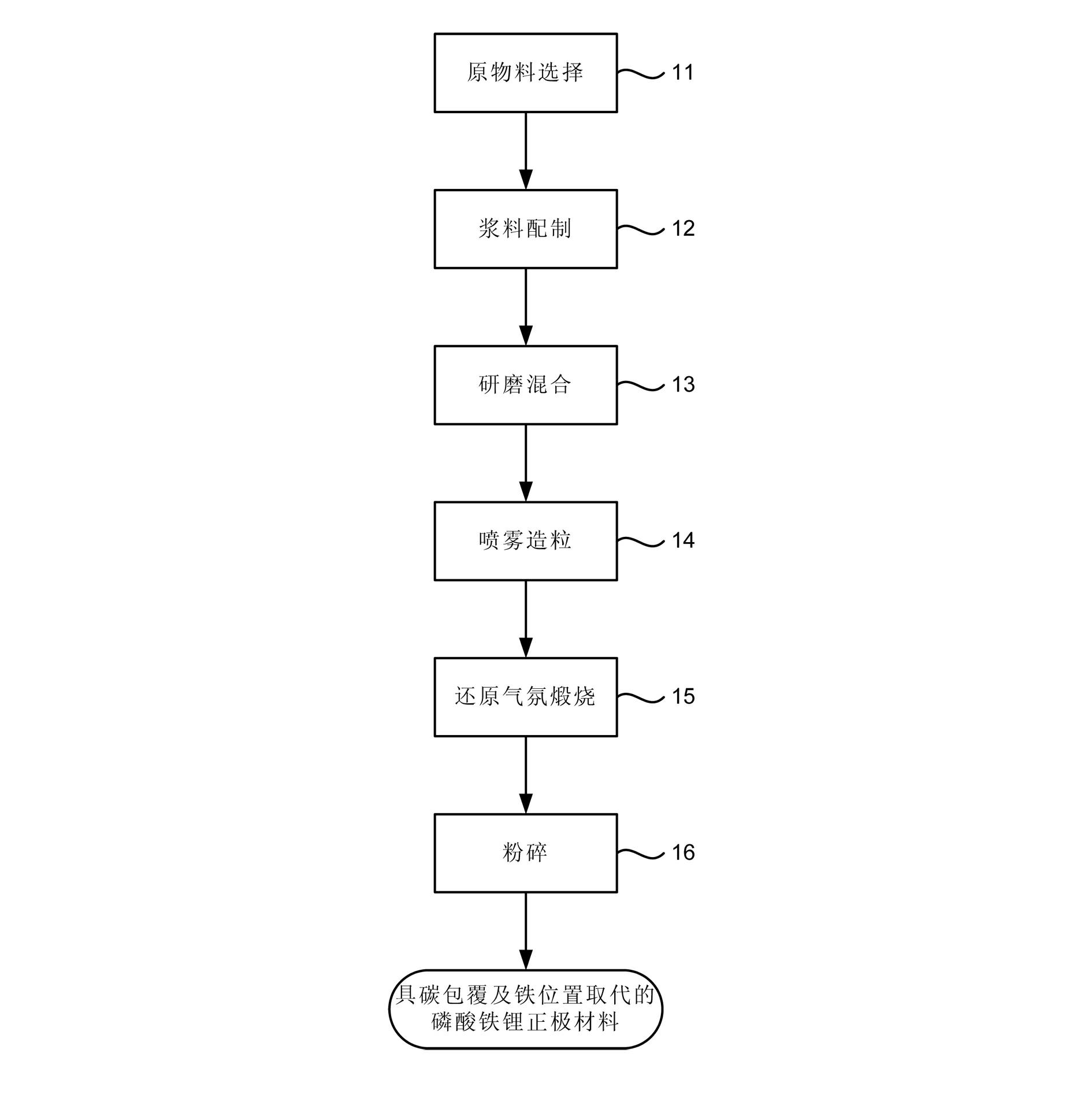
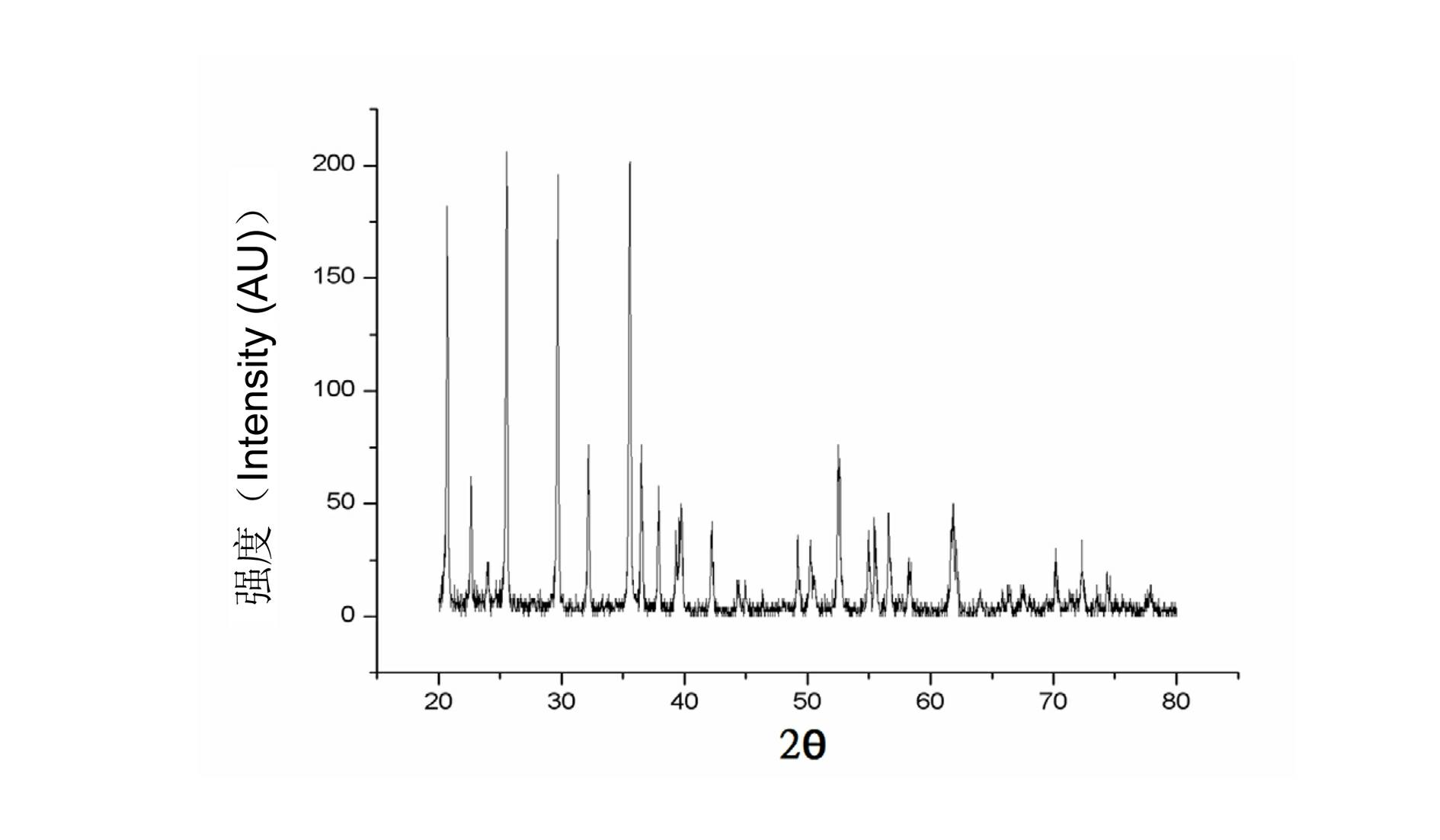
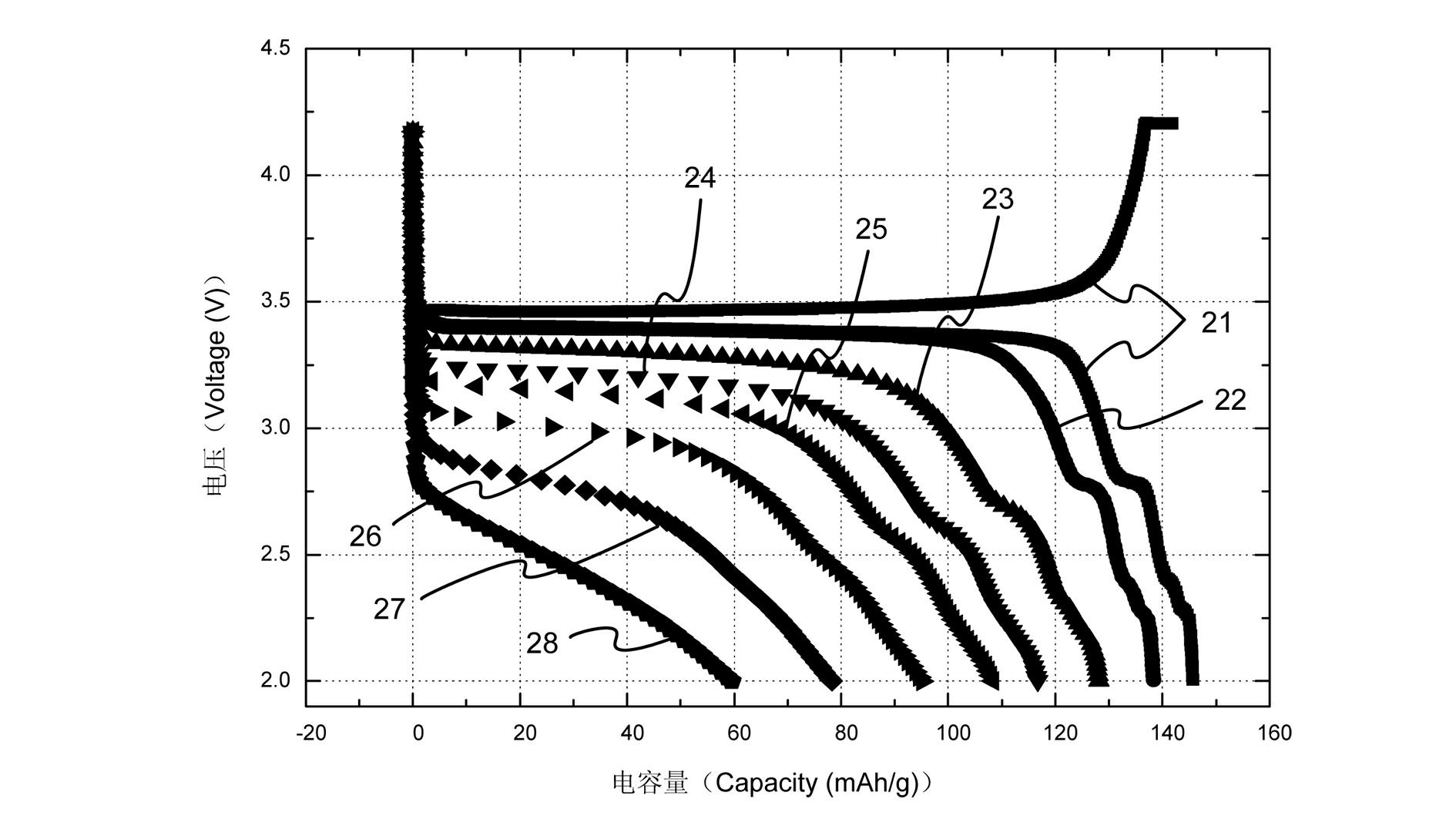
Manufacturing method for lithium iron phosphate cathode material
A lithium iron phosphate and cathode material technology, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve problems such as inability to meet users, and achieve the effects of good charge and discharge life, uniform mixing, and stable quality characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Choose FeC among the compounds containing Li, Fe, P 2 o 4 . 2H 2 O as a water-insoluble compound, LiH 2 PO 4 As a water-soluble compound, zinc oxide (ZnO) is used as the iron position substitution compound (Zn is used as the dopant element replacing iron), and PVP is used as the carbon source. where the LiH 2 PO 4 The preparation includes: first take 3.794kg of lithium carbonate, add 20kg of deionized water, while stirring, add 11.76kg of 85% H 3 PO 4 , by Li 2 CO 3 will be with H 3 PO 4 reaction to generate water-soluble LiH 2 PO 4 and carbon dioxide (CO 2 ). Next, add 0.26Kg of dispersant FN265 and 0.905Kg of carbon source material PVP to the aforementioned aqueous solution, stir together evenly, and then add 17.194Kg of FeC 2 o 4 . 2H 2 O and 0.407Kg of ZnO. Next, grind the aforementioned mixed slurry 4 times with a bead mill to produce a uniformly dispersed ceramic slurry containing Li, Fe, and P elements. At this time, the particle size of the c...
Embodiment 2
[0056] In addition to the material structure mentioned in the above-mentioned embodiments, the method of the present invention can also be the material structure of another embodiment, and the difference is that FeC 2 o 4 . 2H 2 O as a water-insoluble compound, LiH 2 PO 4 As a water-soluble compound, titanium dioxide (TiO 2 ) as the iron position substitution compound (with Ti as the dopant element replacing iron), and ascorbic acid as the carbon source. When preparing, weigh 10.50Kg of LiH 2 PO 4 , dissolved in 18Kg of deionized water, then added 0.28Kg of dispersant BYK180, and 1.80Kg of carbon source material - ascorbic acid, after stirring, then added 16.76Kg of FeC 2 o 4 . 2H 2 O and 0.60kg of TiO 2 . Then, the processes of grinding and mixing, spray granulation, reducing atmosphere calcination, and crushing were carried out as in the above-mentioned Example 1 to produce titanium-containing carbon-coated lithium iron phosphate powder, wherein the condition of ...
Embodiment 3
[0059] In addition to the material structure mentioned in the above-mentioned embodiment, the method of the present invention can also be the material structure of another embodiment, and the difference is that Li 3 PO 4 As a source of water-insoluble Li and P, (CH 3 COO) 2 Fe as a water-soluble iron source, manganese carbonate (MnCO 3 ) as the iron position substitution compound (with Mn as the dopant element replacing iron), and glucose as the carbon source. When preparing, weigh 17.393Kg (CH 3 COO) 2 Fe, dissolved in 22Kg of deionized water, then added 1.6Kg of glucose and 0.32Kg of dispersant 1221, after stirring, then added 10.85Kg of Li 3 PO 4 and 0.92Kg of MnCO 3 . Then, the processes of grinding and mixing, spray granulation, reducing atmosphere calcination, and crushing were carried out as in the above-mentioned Example 1 to produce manganese-containing carbon-coated lithium iron phosphate powder, wherein the condition of reducing atmosphere calcination was si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com