Method for preparing anhydrous magnesium chloride by utilizing magnesium oxide
A technology of anhydrous magnesium chloride and magnesium oxide, which is applied in the direction of magnesium chloride, magnesium halide, etc., can solve the problems of low utilization rate of gas chlorination agent, low utilization rate of gas chlorination agent, and difficult uniform dispersion of gas, so as to reduce environmental protection investment The effect of cost reduction, shortened production process, and easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Mix 3.50g of magnesium oxide and 14.10g of ammonium chloride and add them into a 50ml crucible. Then, 16.00g of sodium chloride was covered on the mixture of magnesium oxide and ammonium chloride, and the crucible was covered with a lid and then kept at 410°C for 1.5 hours, and then at 700°C for 0.5 hours to obtain a layered layer. The upper layer is sodium chloride and the lower layer is anhydrous magnesium chloride. In this embodiment, based on the addition of 1.00 parts by weight of magnesium oxide, the addition of ammonium chloride is 4.03 parts by weight, and the addition of sodium chloride is 4.57 parts by weight.
[0065] The obtained anhydrous magnesium chloride was characterized according to the above measurement method. The result is as follows:
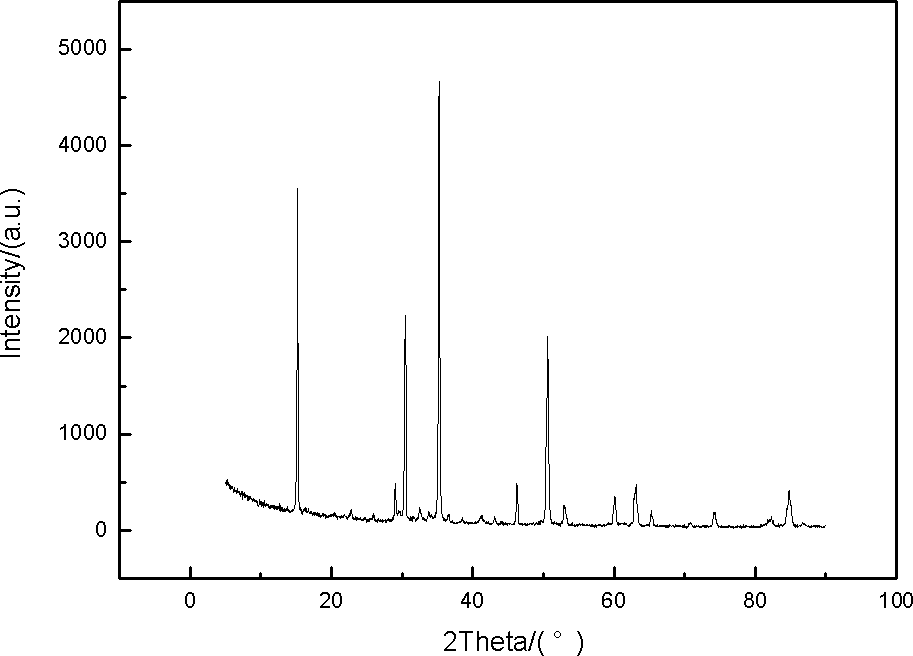
[0066] Carry out XRD phase analysis on the sample composition, the composition is anhydrous MgCl 2 ,Such as figure 1 shown.
[0067] Titrate the magnesium ions and chloride ions in the sample, and the result is ...
Embodiment 2
[0070] Mix 3.50g of magnesium oxide and 28.80g of ammonium chloride and add them into a 50ml crucible. Then, 10.00 g of alumina was covered on the mixture of magnesia and ammonium chloride, and the crucible was covered and kept at a temperature of 410° C. for 1.5 hours, and then at 700° C. for 0.3 hours. In this way, the upper layer of alumina and the lower layer of anhydrous magnesium chloride with obvious layering are obtained. In this embodiment, based on 1.00 parts by weight of magnesium oxide, 8.23 parts by weight of ammonium chloride and 2.86 parts by weight of alumina are added.
[0071] The obtained anhydrous magnesium chloride was characterized according to the above measurement method. The result is as follows:
[0072] Carry out XRD phase analysis on the sample composition, the composition is anhydrous MgCl 2 .
[0073] Titrate the magnesium ions and chloride ions in the sample, and the result is Cl - :Mg 2+ =1.999: 1, the calculated weight of anhydrous magn...
Embodiment 3
[0076] Mix 3.50g of magnesium oxide and 23.50g of ammonium chloride and add them into a 50ml crucible. Then, the crucible was kept covered at a temperature of 410°C for 1.5 hours, and then kept at 700°C for 0.3 hours. In this way, anhydrous magnesium chloride is obtained. In this embodiment, the amount of ammonium chloride added is 6.71 parts by weight based on the amount of magnesium oxide added as 1.00 parts by weight.
[0077] The obtained anhydrous magnesium chloride was characterized according to the above measurement method. The result is as follows:
[0078] Carry out XRD phase analysis on the sample composition, the composition is anhydrous MgCl 2 .
[0079] Titrate the magnesium ions and chloride ions in the sample, and the result is Cl - : Mg 2+ =1.98:1, the calculated weight of anhydrous magnesium chloride is 99.68% of the total mass of the sample; the precipitate after water dissolution in the sample is titrated, and the weight of magnesium oxide is 0.44% of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com