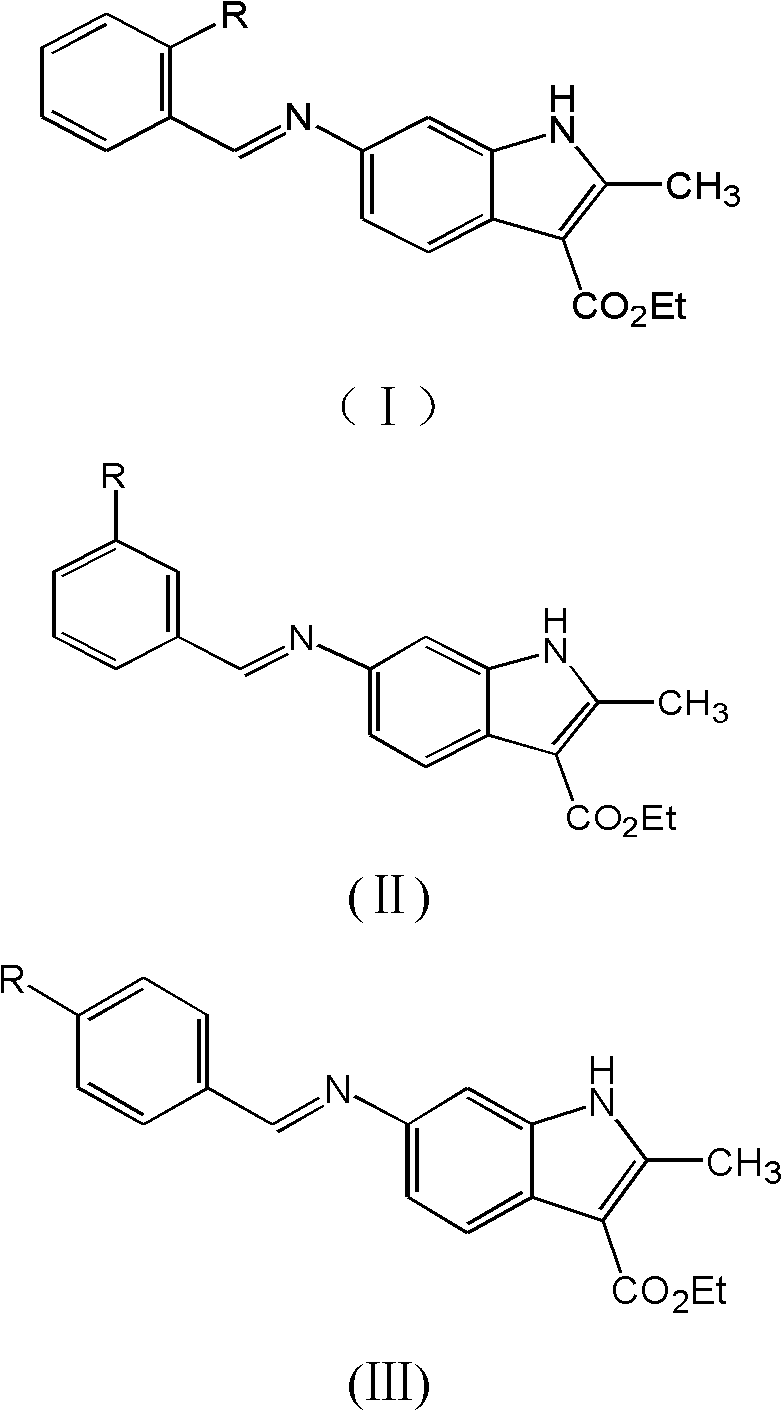
Hydroxyl and alkoxyl benzaldehyde indole Schiff bases, preparation method thereof, and application thereof
A technology of alkoxybenzaldehyde and indole Schiff base, applied in the field of Schiff base preparation, can solve the problems of unfavorable popularization and application, low anticancer activity, low water solubility, etc., achieve low cost, inhibit proliferation, The effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 This embodiment provides the preparation of 2-hydroxybenzaldehyde indole Schiff base:
[0027] Measure 60 mL of ethanol as a solvent in the reactor, add 0.50 g of 2-hydroxybenzaldehyde and 0.9 g of aminoindole compound into the reactor, stir well, heat to reflux, and react for about 6 hours. After the reaction, the solution in the reactor was evaporated to obtain the crude product, and the mixture of ethyl acetate:petroleum ether (60-90° C.)=1:1 was recrystallized to obtain the pure product with a yield of 87.5%.
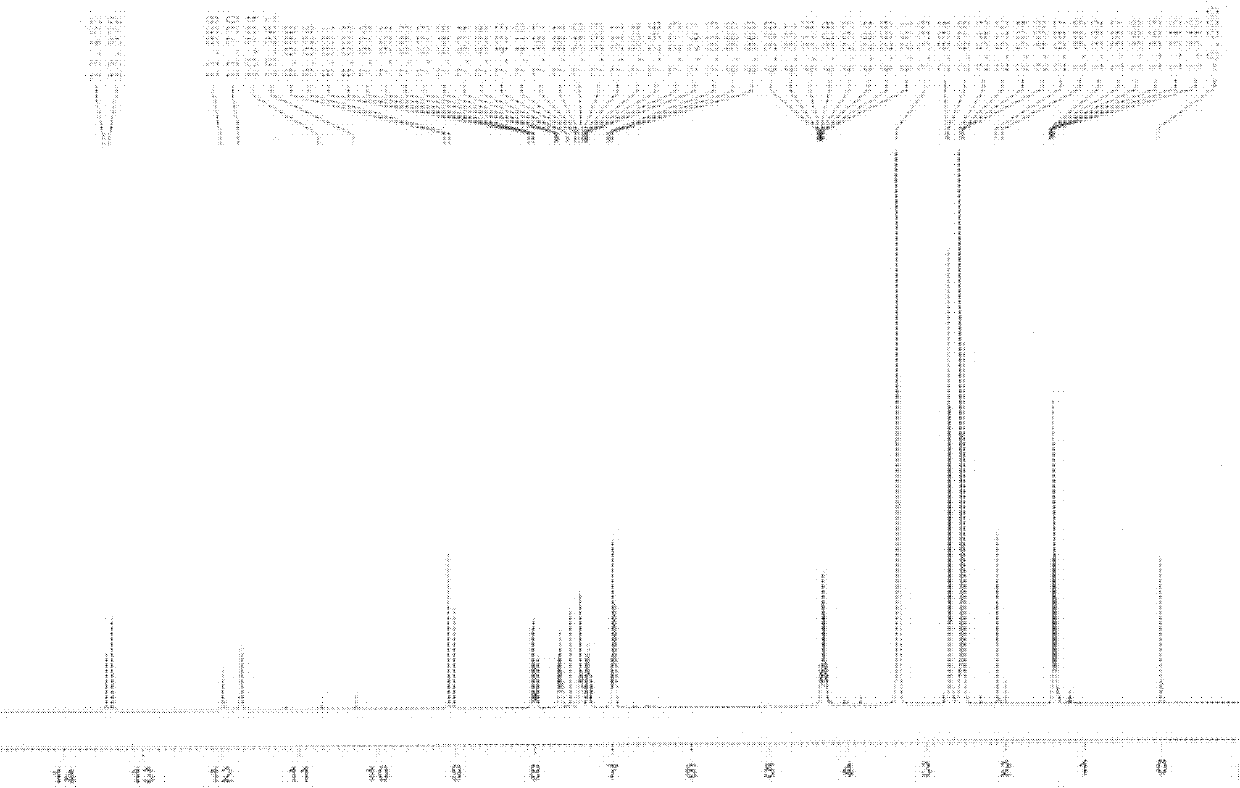
[0028] Hydrogen spectrum of the crystalline product:
[0029] 1 H NMR (300MHz, DMSO-d6, δ): 13.41(s, 1H, OH), 11.84(s, 1H, NH), 9.06(s, 1H, ArH), 8.03(d, 1H, ArH, J=8.7 Hz), 7.95 (d, 1H, ArH, J=8.7Hz), 7.53-6.96 (m, 4H, ArH), 4.29 (q, 2H, CH 2 , J=7.20Hz), 2.68(s, 3H, CH 3 ), 1.36(t, 3H, CH 3 , J=7.20Hz).
[0030] Spectrum such as figure 1 .
Embodiment 2
[0031] Embodiment 2 This embodiment provides the preparation of 3-hydroxybenzaldehyde indole Schiff base:
[0032] Measure 60mL of ethanol as a solvent in the reactor, add 0.62g of 3-hydroxybenzaldehyde and 11g of aminoindole compound into the reactor, stir well, heat to reflux, and react for about 7 hours. After the reaction, the solution in the reactor was evaporated to obtain the crude product, and the pure product was obtained by recrystallization from ethyl acetate:petroleum ether (60-90° C.) = 1:1 mixture with a yield of 92.7%.
Embodiment 3
[0033] Embodiment 3 This embodiment provides the preparation of 4-hydroxybenzaldehyde indole Schiff base:
[0034] Measure 60mL of ethanol as a solvent in the reactor, add 0.73g of 4-hydroxybenzaldehyde and 1.3g of aminoindole compound into the reactor, stir well, heat to reflux, and react for about 5 hours. After the reaction, the solution in the reactor was evaporated to obtain the crude product, and the mixture of ethyl acetate:petroleum ether (60-90° C.)=1:1 was recrystallized to obtain the pure product with a yield of 79.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com