Production method of copolymer of allyl monomer containing polar group
A technology of polar groups and copolymers, which is applied in the field of preparing copolymers of allyl monomers containing polar groups, can solve the problems of reduced polymerization activity, high catalyst cost, and difficulty in use, so as to achieve improved activity, Effect of improving catalyst life and reducing catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Embodiment 1: Copolymerization of allyl acetate and ethylene (preparation of copolymer 1)
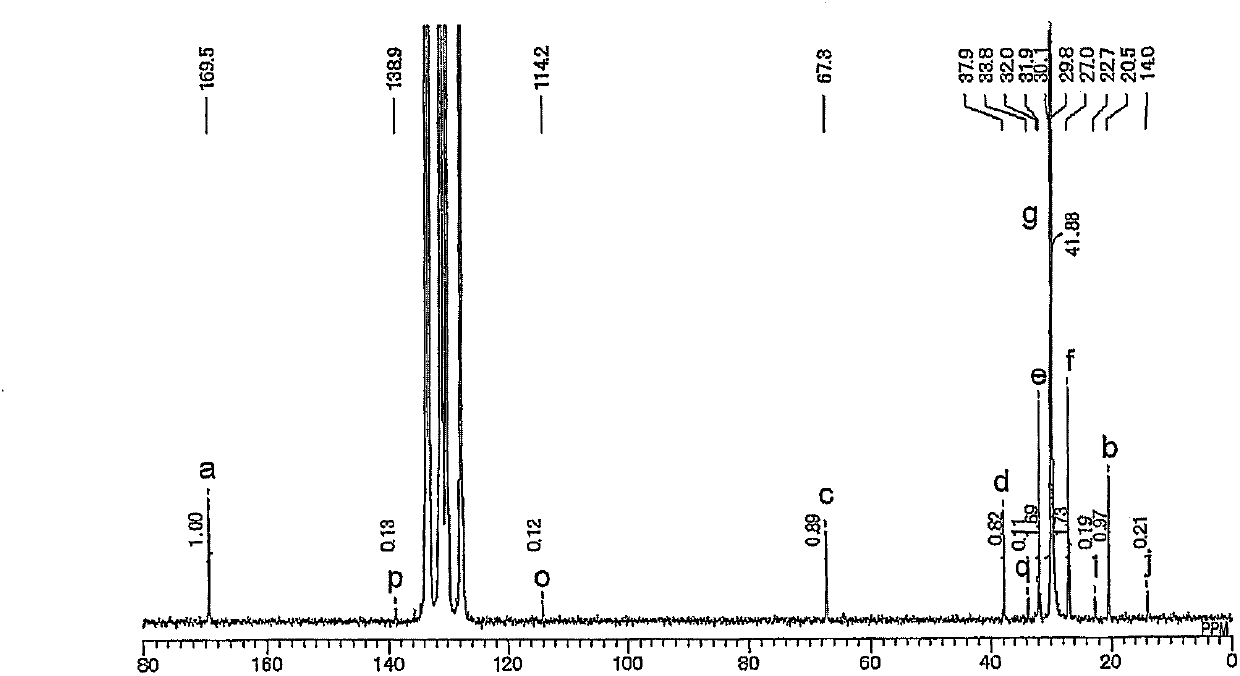
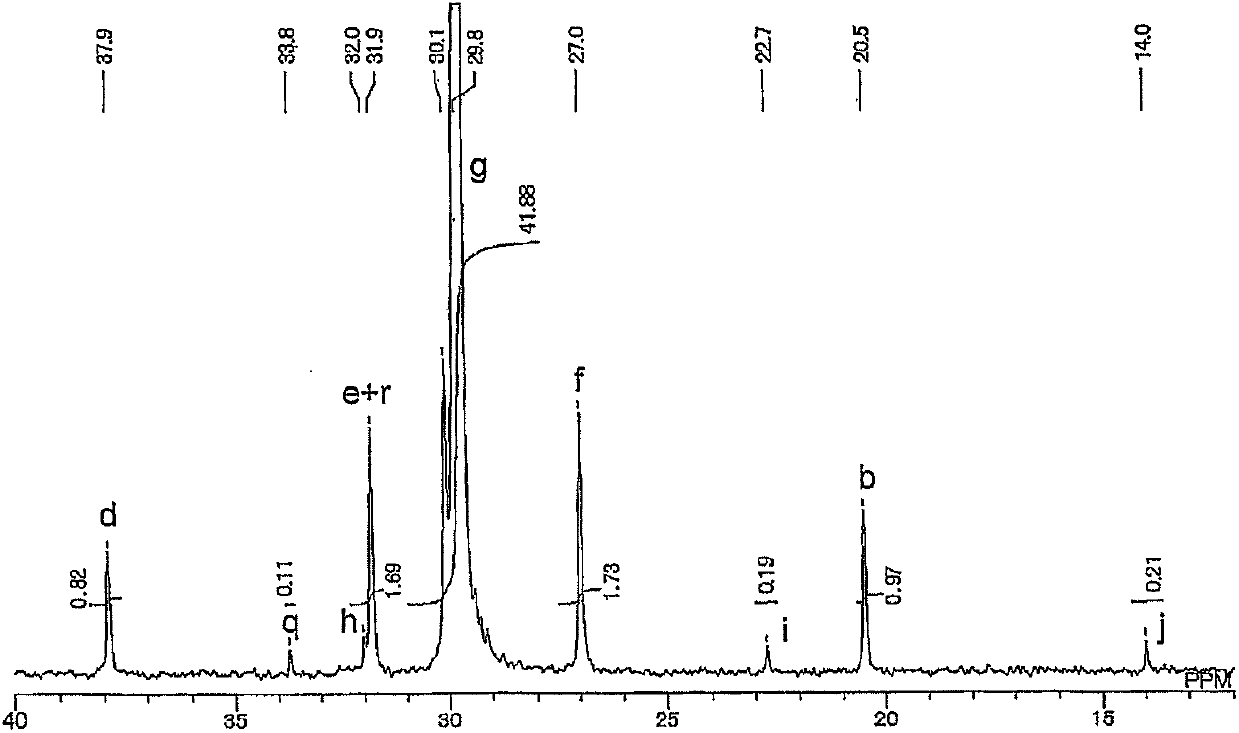
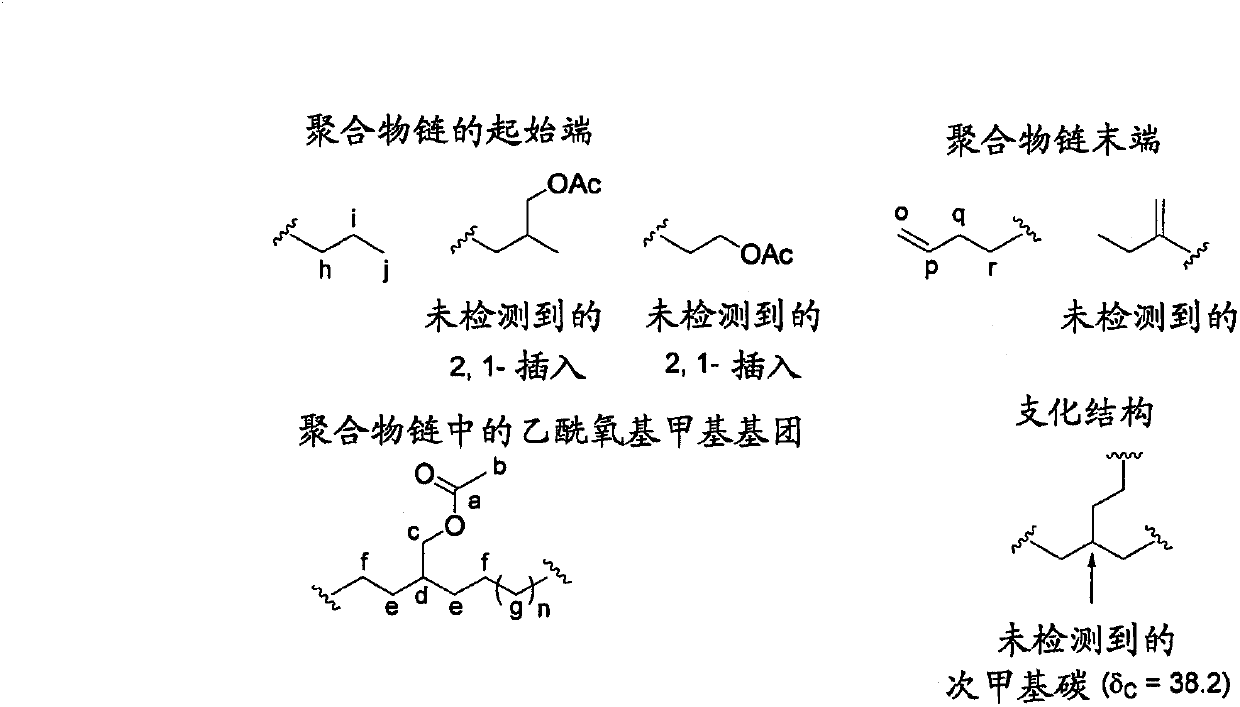
[0230] Under argon atmosphere, dichloromethane (3.75ml), toluene (3.75ml) and allyl acetate (7.5ml, 7.0g, 70mmol) were added to a solution containing metal complex catalyst 1 (58.2g, 0.10mmol). 50ml volume autoclave. After filling the autoclave with ethylene (3.0 MPa), the contents of the autoclave were stirred at 80° C. for 3 hours. After the autoclave was cooled to room temperature, methanol (about 20 ml) was added thereto. The resulting copolymer was recovered by filtration, washed with methanol and dried under reduced pressure to obtain Copolymer 1. Yield 754 mg. By size exclusion chromatography, the number average molecular weight and weight average molecular weight of the copolymer were calculated to be 8,100 and 16,200, respectively, and Mw / Mn was 2.0. By using the inverse gating decoupling method of the 13 The allyl acetate content of the copolymer was determined by ...
Embodiment 2
[0235] Embodiment 2: the copolymerization of allyl acetate and ethylene (preparation copolymer 2)
[0236] Toluene (7.5 ml) and allyl acetate (7.5 ml, 7.0 g, 70 mmol) were added to a 50 ml volume autoclave containing metal complex catalyst 1 (58.2 g, 0.10 mmol) under an argon atmosphere. After filling the autoclave with ethylene (3.0 MPa), the contents of the autoclave were stirred at 80° C. for 3 hours. After the autoclave was cooled to room temperature, methanol (about 20 ml) was added thereto. The resulting copolymer was recovered by filtration, washed with methanol and dried under reduced pressure to obtain Copolymer 2. Yield was 585 mg. By size exclusion chromatography, the number average molecular weight and weight average molecular weight of the copolymer were calculated to be 7,900 and 15,500, respectively, and Mw / Mn was 2.0. By using the inverse gating decoupling method of the 13 The allyl acetate content of the copolymer was determined by C-NMR spectrum, and the ...
Embodiment 3
[0237] Embodiment 3: the copolymerization of allyl acetate and ethylene (preparation copolymer 3)
[0238] Metal complex catalyst 2 was synthesized in the same manner as metal complex catalyst 1 using 2-(dicyclopentylphosphino)benzenesulfonic acid as a starting material.
[0239]
[0240] Cyp = cyclopentyl
[0241] Using the obtained metal complex catalyst 2, the copolymerization of allyl acetate and ethylene was carried out in the same manner as in Example 2. That is, under an argon atmosphere, toluene (7.5 ml) and allyl acetate (7.5 ml, 7.0 g, 70 mmol) were added to a 50 ml volume autoclave containing metal complex catalyst 2 (0.10 mmol). After filling the autoclave with ethylene (3.0 MPa), the contents of the autoclave were stirred at 80° C. for 3 hours. After the autoclave was cooled to room temperature, methanol (about 20 ml) was added thereto. The resulting copolymer was recovered by filtration, washed with methanol and dried under reduced pressure to obtain copoly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com