Drug combination, as well as preparation method and application of same
A composition and medicine technology, applied in the preparation of the above-mentioned pharmaceutical composition, the pharmaceutical composition containing reserpine, and the application field of the above-mentioned pharmaceutical composition, can solve the change of the appearance and color of the tablet, the decrease of the drug content, the unsatisfactory results, etc. problem, to achieve the effect of low price, improved stability and exact curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Reserpine (1 original drug), hydrochlorothiazide (2 original drugs), promethazine hydrochloride (3 original drugs), dihydralazine sulfate (4 original drugs), vitamin B 6 (5 active ingredients), vitamin B 1 (6 original drug) and potassium dihydrogen phosphate and other acidic substances were mixed in a certain proportion and then granulated. The granules were subjected to strong light (4500Lx), high temperature (60°C) and high humidity (RH75%) influencing factors. Take samples on day 5 and 10, detect the drug content, and compare the results with day 0. The results are shown in Table 2.
[0050]
[0051]
[0052] Table 2 Reserpine drug and each adjuvant influencing factors test drug content change (%)
[0053] The results of investigation on the influencing factors of each original drug showed that the stability of the drug was greatly improved after the addition of potassium dihydrogen phosphate. However, the stability of dihydralazine sulfate combined with citr...
Embodiment 2
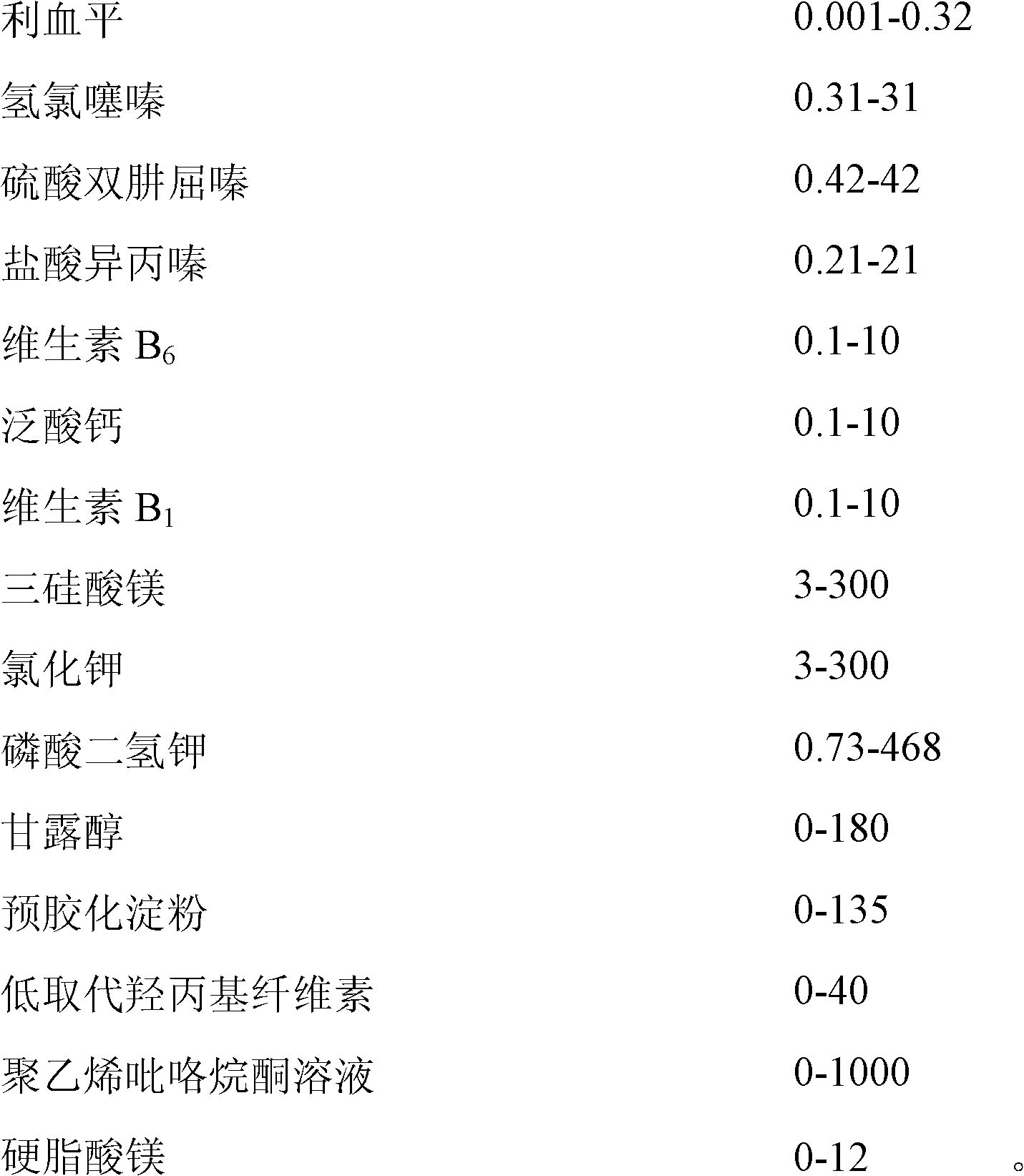
[0055] The present embodiment is a pharmaceutical composition containing reserpine. Based on 1000 tablets, the weight of the main drug and auxiliary ingredients is as follows:
[0056]
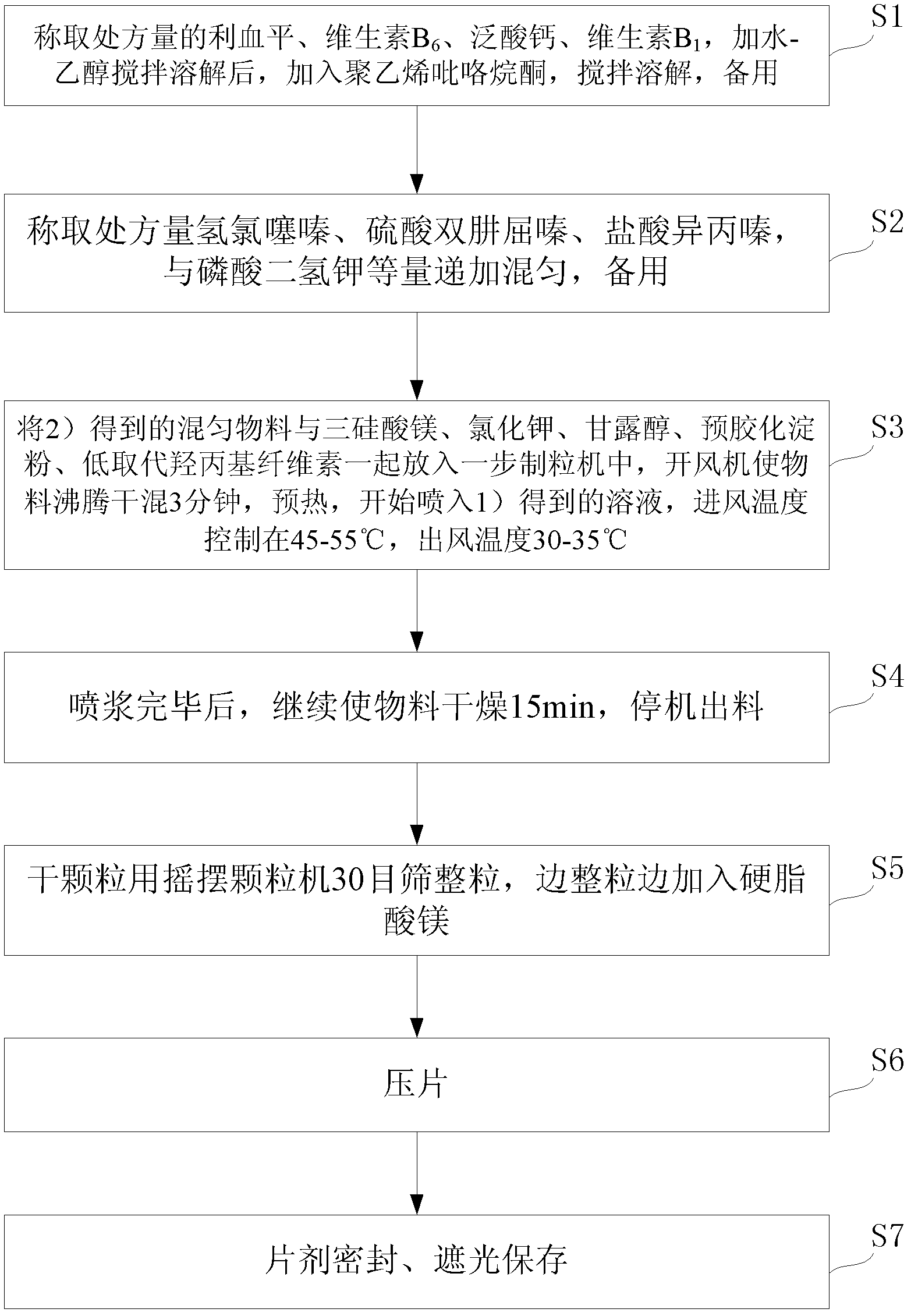
[0057] see figure 1 , taking the preparation of 1000 tablets as an example, the preparation method of the above-mentioned pharmaceutical composition preparation containing reserpine is as follows:
[0058] Step S1) weigh the reserpine and vitamin B of the prescription amount 6 , calcium pantothenate, vitamin B 1 , add water-ethanol and stir to dissolve, add polyvinylpyrrolidone, stir to dissolve, set aside;
[0059] Step S2) Weigh the prescribed amount of hydrochlorothiazide, dihydralazine sulfate, and promethazine hydrochloride, add and mix with potassium dihydrogen phosphate in equal amounts, and set aside;
[0060] Step S3) put the mixed material obtained in step S2) into a one-step granulator together with magnesium trisilicate, potassium chloride, mannitol, pregelatinized starch, a...
Embodiment 3
[0074] The present embodiment is a pharmaceutical composition containing reserpine, capsule. Based on 1000 capsules, the weight of main ingredients and auxiliary materials is as follows:
[0075]
[0076]
[0077] Taking the preparation of 1000 tablets as an example, the preparation method of the above-mentioned pharmaceutical composition preparation containing reserpine is as follows:
[0078] 1) Weigh the prescription amount of reserpine and vitamin B 6 , calcium pantothenate, vitamin B 1 After adding water-ethanol and stirring to dissolve, add polyvinylpyrrolidone and phosphoric acid, stir to dissolve, and set aside;
[0079] 2) Weigh the prescribed amount of hydrochlorothiazide, dihydralazine sulfate, and promethazine hydrochloride, and mix them with pregelatinized starch in equal amounts, and set aside;
[0080] 3) Put the mixed material obtained in 2) into a one-step granulator together with magnesium trisilicate, potassium chloride, microcrystalline cellulose, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com