Zinc-aluminum binary hydrotalcite and application thereof as photocatalytic material used for degrading methyl violet
A catalytic material, hydrotalcite technology, applied in zinc oxide/zinc hydroxide, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as impact, achieve high dye removal rate, mild reaction conditions, and easy recycling The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 zinc-aluminum binary hydrotalcite
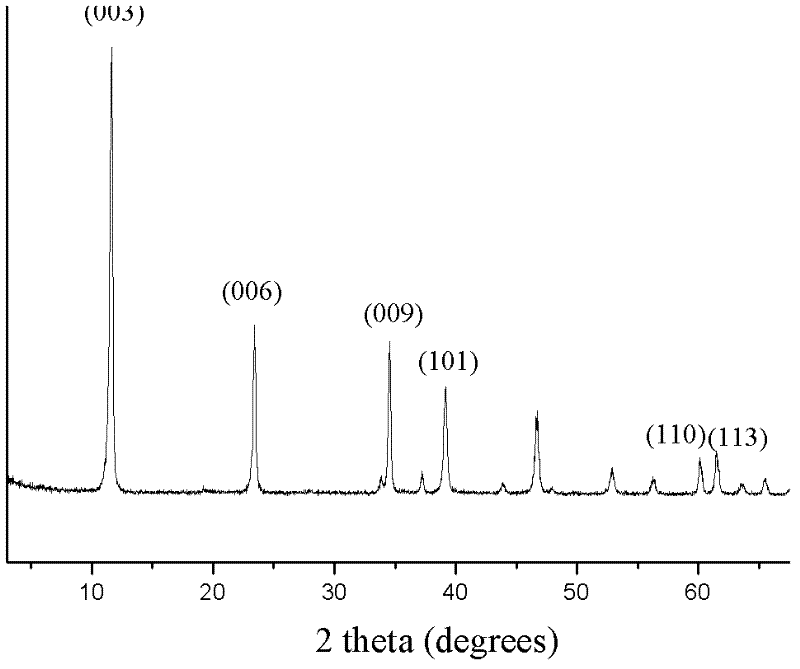
[0035] Take Zn(NO 3 ) 2 ·6H 2 O(0.3mol) and Al(NO 3 )3 9H 2 O (0.1mol) was dissolved in 300ml water to prepare solution A, 0.8molNaOH and 0.05molNa 2 CO 3 Dissolve in 300ml water to prepare solution B, add solution A and solution B dropwise to 100ml deionized water respectively, keep the dropping rate of 1 drop / second, stir vigorously at a constant temperature of 40°C, and keep the pH value between 9 and 10 After the dropwise addition, continue to stir for 60 minutes, crystallize at 65°C for 18h, centrifuge, wash the precipitate with deionized water until neutral, dry at 85°C for 12h to obtain a zinc-aluminum binary hydrotalcite (ZnAl-LDHs) sample, X-ray diffraction Figure (XRD) as figure 1 As shown, the ratio of zinc to aluminum is 3:1, that is, x is 0.25, m is 4, and the general chemical formula is
[0036] [Zn 2+ 0.75 Al 3+ 0.25 (OH) 2 ](CO 3 2- ) 0.125 4H 2 O].
Embodiment 2
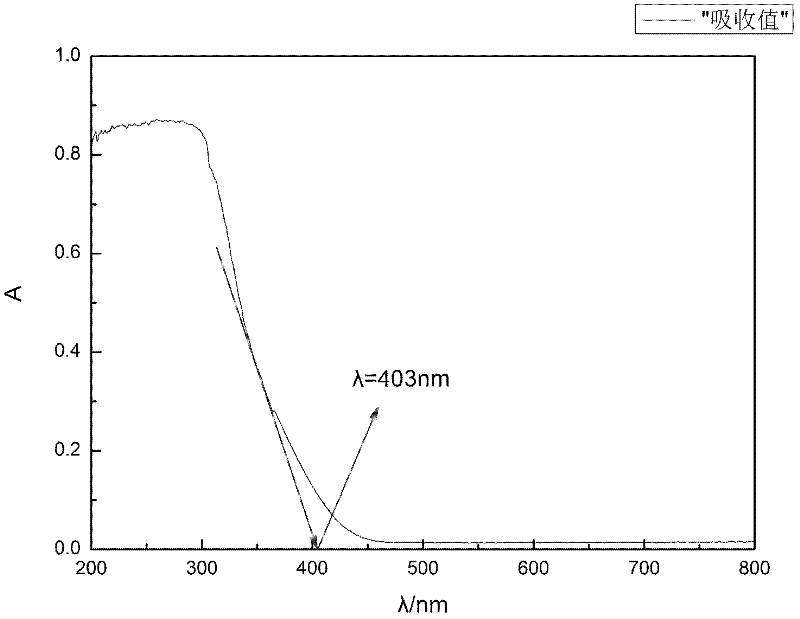
[0038] Get 2000mg of zinc-aluminum binary hydrotalcites with a zinc-aluminum ratio of 3:1 prepared by the method in Example 1, scan the 200-800nm wavelength range in a UV-visible spectrophotometer (type 2550, Shimadzu), and measure the zinc-aluminum binary water. Diffuse reflectance spectrum of talc, see figure 2 , according to the absorption edge of the measured spectrum, the absorption edge wavelength is obtained, and then the band gap energy is calculated according to the formula Eg=1240 / λg (Eg is the band gap energy, and λg is the absorption edge wavelength).
[0039] Conclusion: The bandgap energy of zinc-aluminum binary hydrotalcite is 3.05eV, and a dysprosium lamp can be chosen as the excitation light source.
Embodiment 3
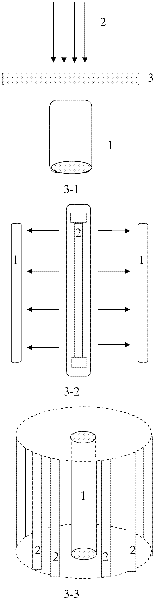
[0041] Prepare 3 servings of 50ml 20mg·L -1 For the methyl violet aqueous solution, three parts of the zinc-aluminum binary hydrotalcite prepared by the method in Example 1 were weighed, 100 mg in each part, respectively added to the above-mentioned methyl violet aqueous solution to be treated (3 groups were formed), and the temperature was controlled at 25 ° C. pH=7, respectively placed in three different reaction models ( image 3 Among them, 3-1 is the parallel reaction model of external illumination; 3-2 is the reaction model of internal illumination; 3-3 is the ring reaction model), and the irradiation time of the dysprosium lamp is controlled to be 1h. After stirring, the three groups of solutions are centrifuged at 2000rpm for 10min, respectively. Get supernatant liquid and measure the absorbance of methyl violet at 590nm place, calculate the concentration of methyl violet according to methyl violet standard curve, according to formula (1) and (2), obtain the zinc-alumi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Band gap energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com