Preparation method of magnetic iron oxide nanoparticle capable of stably dispersing in water
A magnetic iron oxide and nanoparticle technology, applied in the fields of biomaterials, nanoscience, and material chemistry, can solve the problems of low particle stability and incomplete replacement, and achieve a simple and controllable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
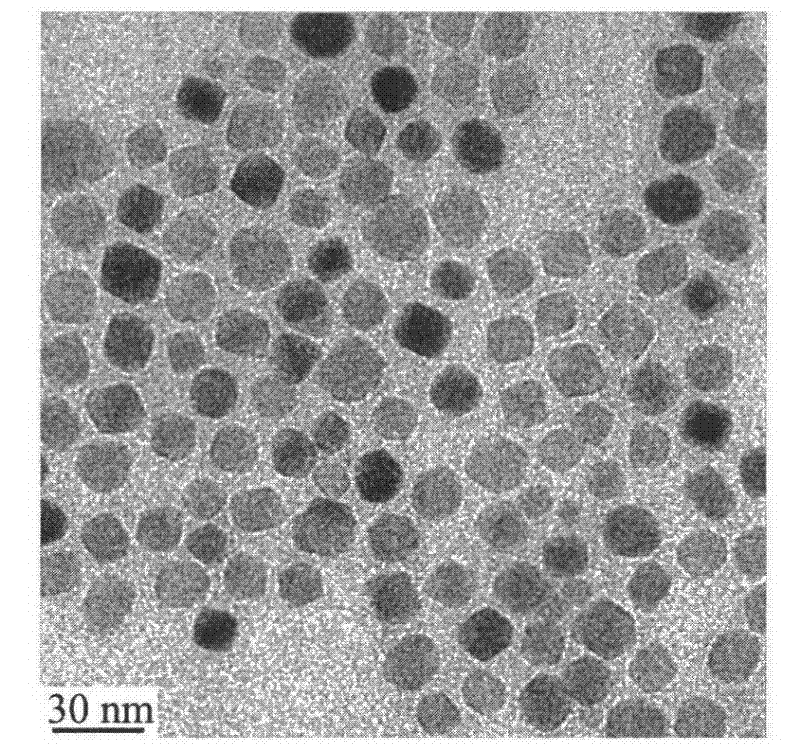
[0018] Weigh 15.1 g of PEG (molecular weight: 1000), and add 0.11 g of PEI (molecular weight: 1800). Put the above raw materials into a three-necked flask, place it on a temperature-controlled magnetic stirrer and heat it to 80°C, then add 0.72 g of analytically pure iron acetylacetonate, stir with a magnetic stirrer for 10 minutes, and protect it with flowing argon during the heating process. Then the temperature was raised to 260° C. and heated for 60 minutes, the solution changed from red to black. After stopping the heating, wait for the solution to cool below 60°C, add 60ml of analytically pure toluene, separate the black precipitate by magnetic adsorption, wash twice with analytically pure acetone to remove excess organic matter, and dissolve the precipitate in water. The electrophoretic particle size of the nanoparticles is 28nm. The morphology of the nanoparticles was observed with a transmission electron microscope (TEM). figure 1 It is a transmission electron micro...
Embodiment 2
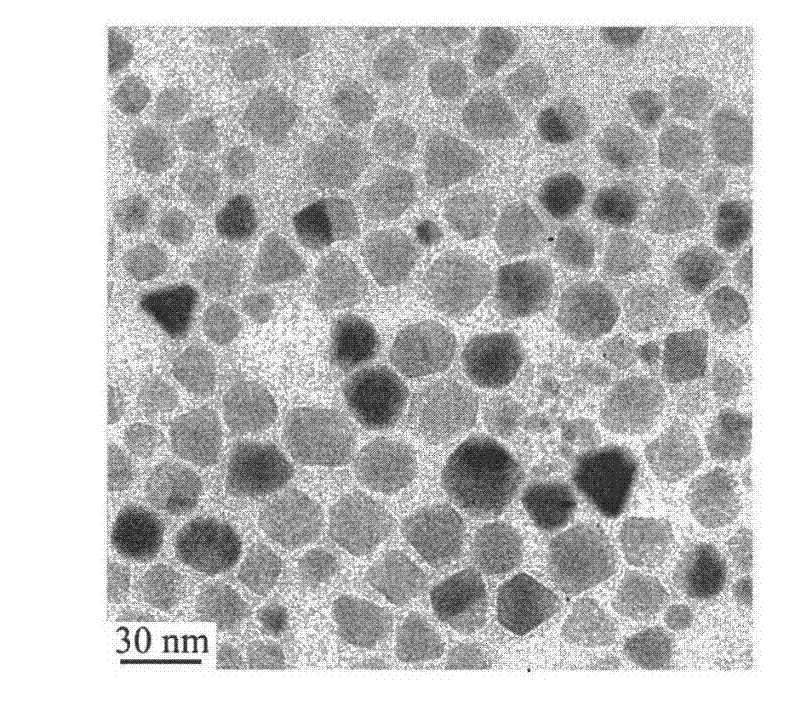
[0020] 15.3 g of PEG (molecular weight: 1000) was weighed, and 0.31 g of PEI (molecular weight: 1800) was added. Put the above raw materials into a three-necked flask, place it on a temperature-controlled magnetic stirrer and heat it to 80°C, then add 0.72 g of analytically pure iron acetylacetonate, stir with a magnetic stirrer for 10 minutes, and protect it with flowing argon during the heating process. Then the temperature was raised to 260° C. and heated for 60 minutes, the solution changed from red to black. After stopping the heating, wait for the solution to cool below 60°C, add 60ml of analytically pure toluene, separate the black precipitate by magnetic adsorption, wash twice with analytically pure acetone to remove excess organic matter, and dissolve the precipitate in water. The average electrophoretic particle size of the nanoparticles is 27nm. The morphology of the nanoparticles was observed with a transmission electron microscope (TEM). figure 2It is a transmi...
Embodiment 3
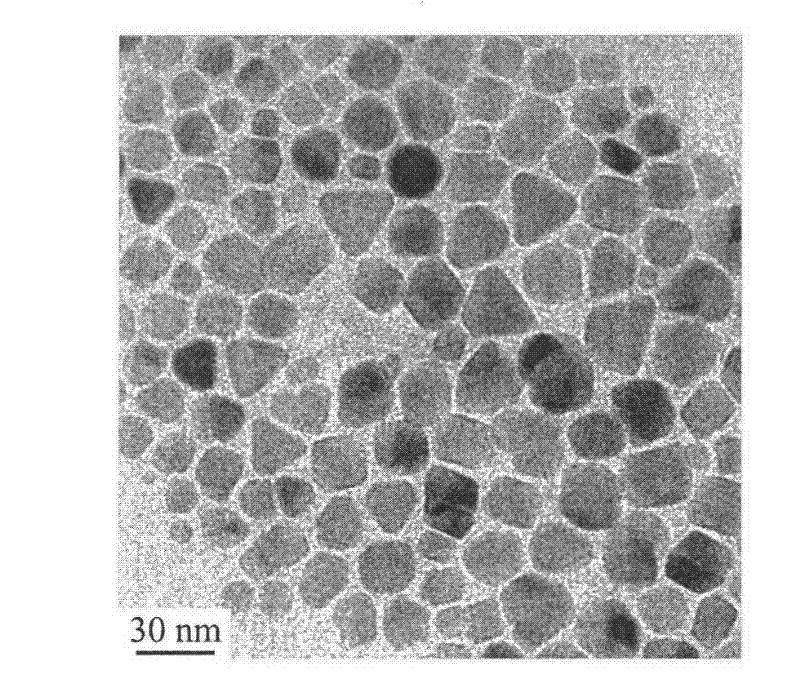
[0022] 15.2 g of PEG (molecular weight: 1000) was weighed, and 0.51 g of PEI (molecular weight: 1800) was added. Put the above raw materials into a three-necked flask, place it on a temperature-controlled magnetic stirrer and heat it to 80°C, then add 0.73 g of analytically pure iron acetylacetonate, stir with a magnetic stirrer for 10 minutes, and protect it with flowing argon during the heating process. Then the temperature was raised to 260° C. and heated for 60 minutes, the solution changed from red to black. After stopping the heating, wait for the solution to cool below 60°C, add 60ml of analytically pure toluene, separate the black precipitate by magnetic adsorption, wash twice with analytically pure acetone to remove excess organic matter, and dissolve the precipitate in water. The average electrophoretic particle size of the nanoparticles is 30nm. The morphology of the nanoparticles was observed with a transmission electron microscope (TEM). image 3 It is a transmi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com