Multidentate pyridine ligand lanthanum metal coordination compound and preparation method thereof
A metal complex, multidentate pyridine technology, applied in the field of multidentate pyridine ligand lanthanum metal complex and its preparation, can solve the problems of high reaction temperature, poor repeatability, high risk, etc., and achieves mild reaction conditions, High productivity and improved energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of the multidentate pyridine ligand lanthanum metal complex, the steps are as follows:
[0023] 1) At room temperature, 70 mg of the ligand 2,3,6,7,10,11-hexa(2-pyridine)bispyrazine[2,3-f:2′,3′-h]quinoxaline HPDQ ( 0.1mmol) was dissolved in 10mL of dichloromethane, and the ligand solution was obtained after stirring evenly;
[0024] 2) At room temperature, the La(NO 3 ) 3 ·6H 2 O 130 mg (0.3 mmol) 3 Dissolve in 10mL of acetonitrile, stir well to obtain a metal salt solution;
[0025] 3) At room temperature, add 10 mL of HPDQ ligand solution to 10 mL of lanthanum nitrate metal salt solution to obtain a reaction solution, and stir for 2 hours;
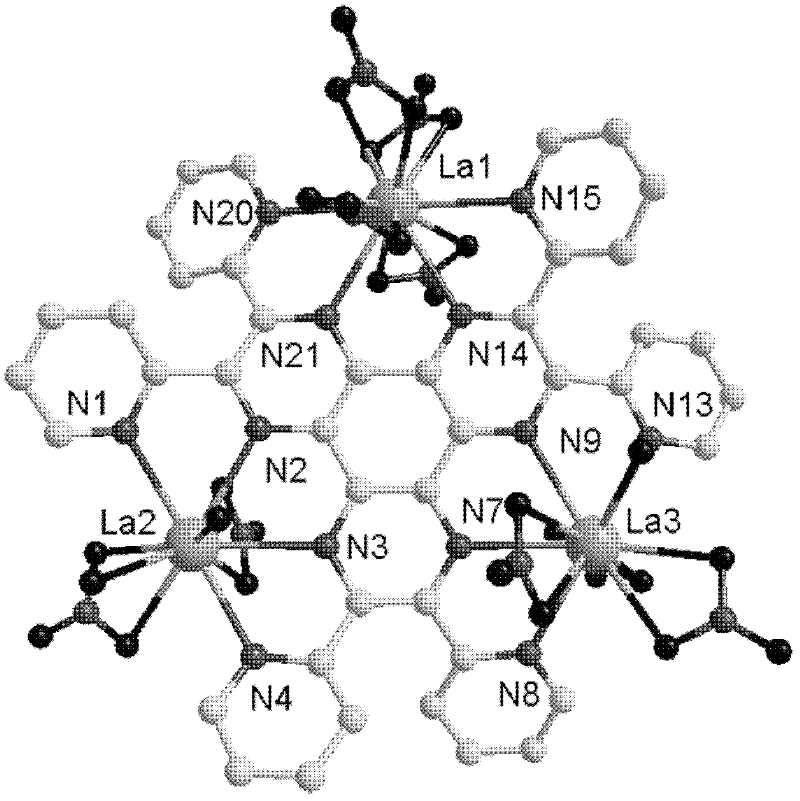
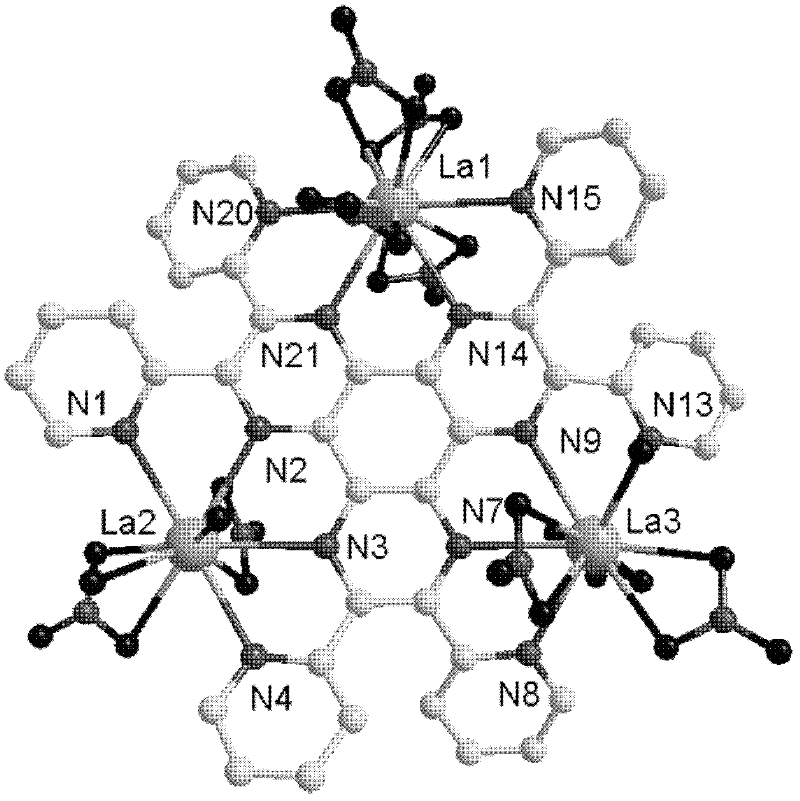
[0026] 4) At room temperature, filter the reaction solution, and let the filtrate stand for 48 days to obtain a dark yellow massive single crystal, which is 2,3,6,7,10,11-hexa(2-pyridine)bispyrazine[2,3 -f: Lanthanum metal complexes of 2',3'-h]quinoxaline ligands.
Embodiment 2
[0028] A preparation method of the multidentate pyridine ligand lanthanum metal complex, the steps are as follows:
[0029] 1) At room temperature, 140 mg of the ligand 2,3,6,7,10,11-hexa(2-pyridine)bispyrazine[2,3-f:2′,3′-h]quinoxaline HPDQ ( 0.2mmol) was dissolved in 10mL of chloroform, and the ligand solution was obtained after stirring evenly;
[0030] 2) At room temperature, the La(NO 3 ) 3 ·6H 2 O 260mg (0.6mmol) 3 Dissolve in 10mL propionitrile, stir well to obtain a metal salt solution;
[0031] 3) At room temperature, add 10 mL of HPDQ ligand solution to 10 mL of lanthanum nitrate metal salt solution to obtain a reaction solution, and stir for 2 hours;
[0032] 4) At room temperature, filter the reaction solution, and let the filtrate stand for 43 days to obtain a dark yellow massive single crystal, which is 2,3,6,7,10,11-hexa(2-pyridine)bispyrazine[2,3 -f: Lanthanum metal complexes of 2',3'-h]quinoxaline ligands.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com