Preparation method of pectinate high-polymer phase-change energy storage material
A phase change energy storage material and polymer technology, which can be used in heat exchange materials, chemical instruments and methods, etc., and can solve problems such as decreased processability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
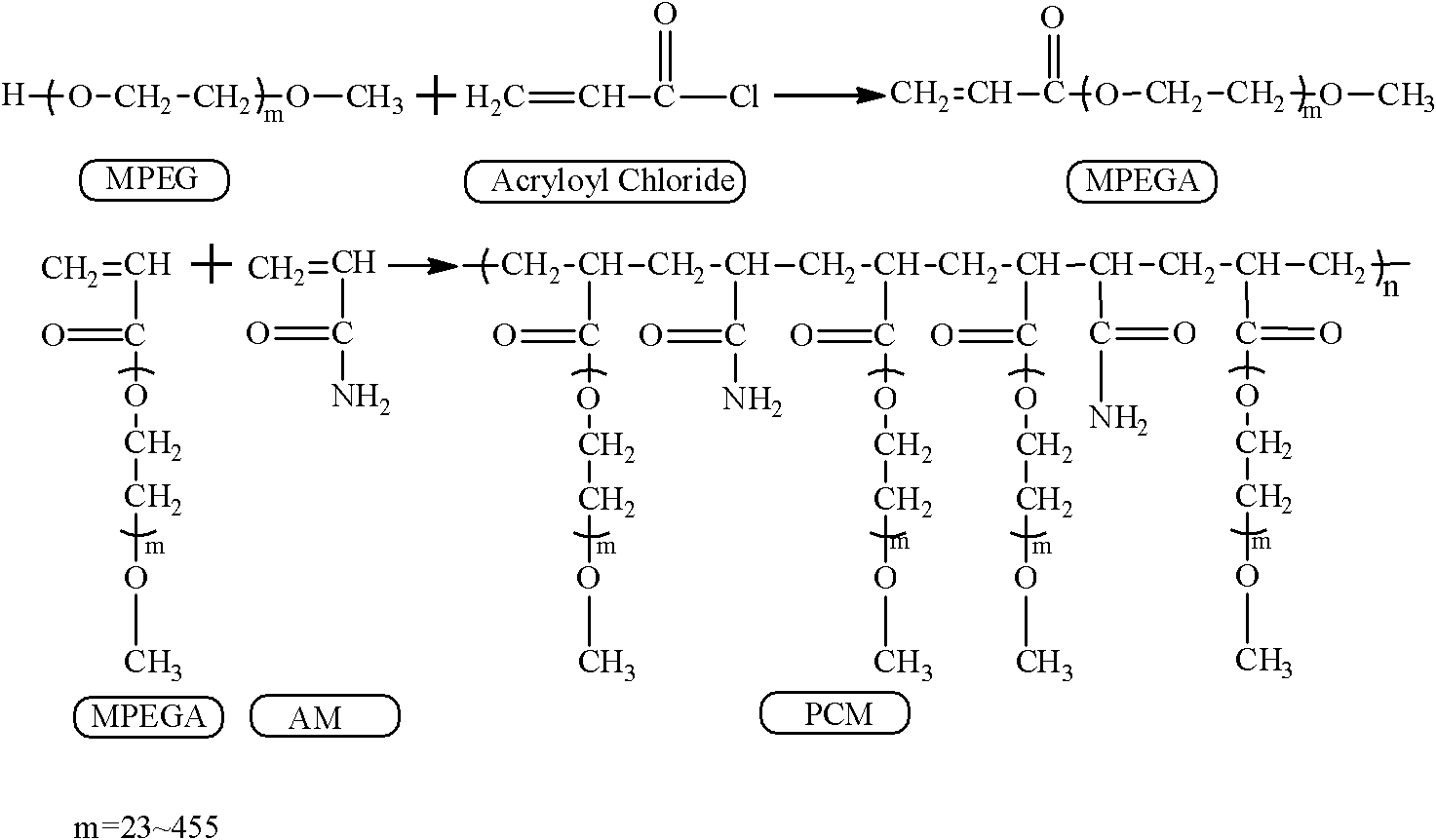
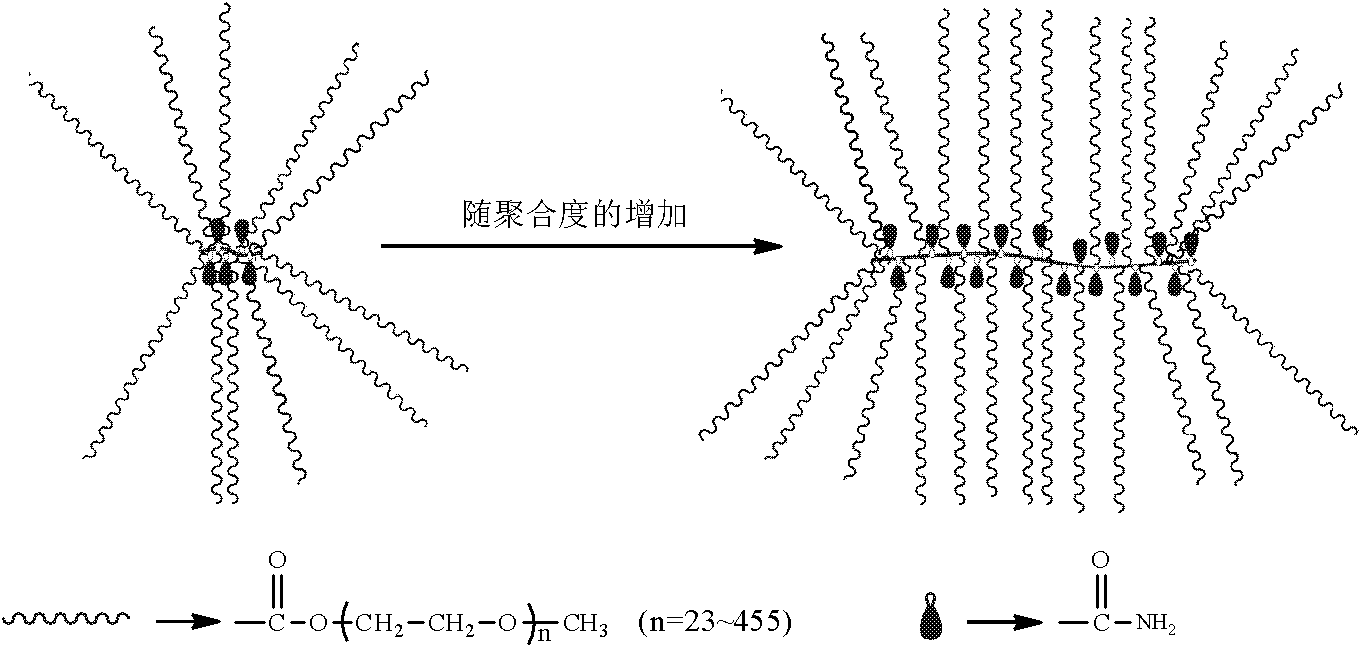
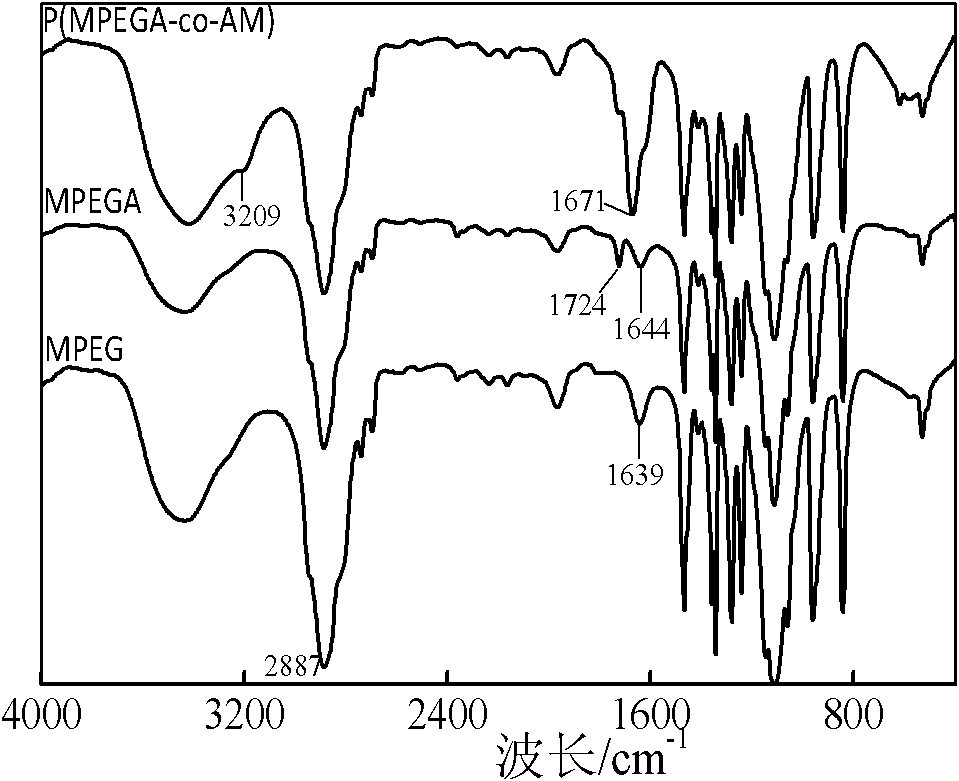
[0033] In an ice-water bath, dissolve 50 g of polyethylene glycol monomethyl ether (MPEG) with a molecular weight of 2000 in 100 ml of dichloromethane, add 0.663 g of acid-binding agent sodium carbonate, and add 2.03 ml of acryloyl chloride to the system after a period of nitrogen gas flow; The temperature of the system was raised to 35°C, and reacted for 8 hours under the protection of nitrogen; under an ice-water bath, anhydrous ether was added to the reaction solution, allowed to stand for 10 minutes, and filtered under reduced pressure to obtain polyethylene glycol monomethyl ether acrylate; Dissolve 2g of glycol monomethyl ether ester and 0.080g of acrylamide in 2ml of water, add 0.020g of ammonium persulfate, and react in water at 75°C for 40 minutes to obtain a phase-change energy storage material with a comb-like molecular chain shape. The preparation reaction formula is shown in figure 1 . From the structural diagram ( figure 2 ) It can be seen that when the degree...
Embodiment 2
[0038]In an ice-water bath, dissolve 50 g of polyethylene glycol monomethyl ether (MPEG) with a molecular weight of 1000 in 100 ml of dichloromethane, add 2.650 g of acid-binding agent sodium carbonate, and add 4.06 ml of acryloyl chloride to the system after passing nitrogen for a period of time; The temperature of the system was raised to 40°C, and reacted for 6 hours under the protection of nitrogen; under an ice-water bath, add n-hexane to the reaction solution, let it stand for 15 minutes, and filter under reduced pressure to obtain polyethylene glycol monomethyl ether acrylate; Dissolve 2g of alcohol monomethyl ether ester and 0.240g of N-isopropylacrylamide in 1ml of water-ethanol (v / v=5 / 95) composite solvent, add 0.020g of azobisisobutyronitrile, and dissolve in 70°C water After reacting for 30 minutes, a phase-change energy storage material with comb-like molecular chain morphology was obtained.
Embodiment 3
[0039] Example 3 [0038] Example 3
[0040] In an ice-water bath, dissolve 50 g of polyethylene glycol monomethyl ether (MPEG) with a molecular weight of 1000 in 100 ml of dichloromethane, add 1.325 g of acid-binding agent sodium carbonate, and add 8.12 ml of acryloyl chloride to the system after passing nitrogen for a period of time; The temperature of the system was raised to 35°C, and reacted for 8 hours under the protection of nitrogen; under an ice-water bath, diethyl ether was added to the reaction solution, allowed to stand for 15 minutes, and filtered under reduced pressure to obtain polyethylene glycol monomethyl ether acrylate; polyethylene glycol acrylic acid Dissolve 2g of monomethyl ether ester and 0.080g of acrylamide in 1ml of water, add 0.020g of ammonium persulfate, and react in water at 85°C for 120min to obtain a phase-change energy storage material with a comb-like molecular chain shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com