Method for fixing terpyridyl ruthenium on surface of electrochemical electrode
A technology of ruthenium terpyridine and electrode surface, which is applied in the fields of electrochemical variables of materials, chemiluminescence/bioluminescence, and analysis by making materials undergo chemical reactions, etc. It can solve the problems of poor stability, cumbersome operation, and poor conductivity of electrodes, etc. problems, to achieve the effect of broad application prospects, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
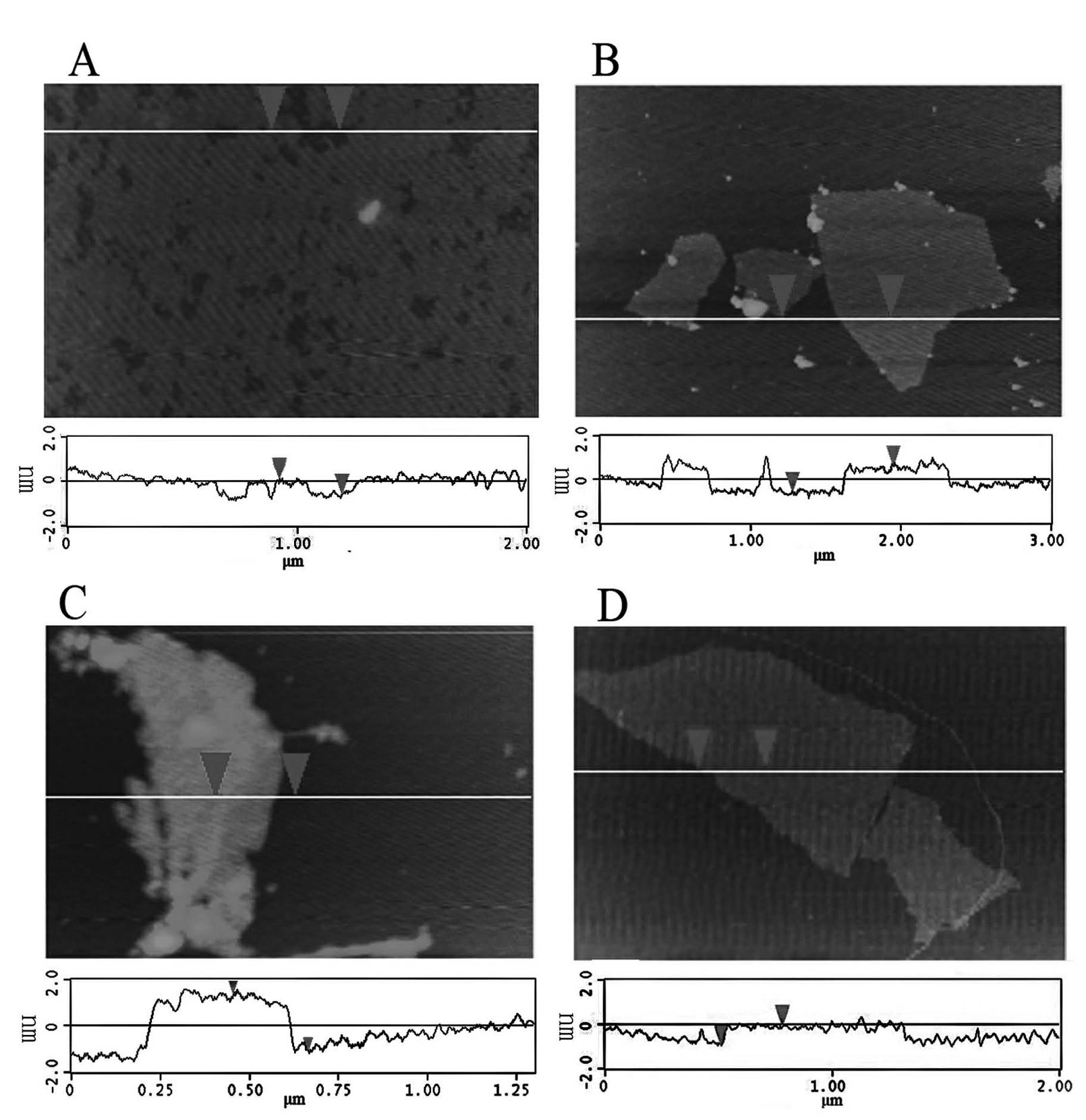
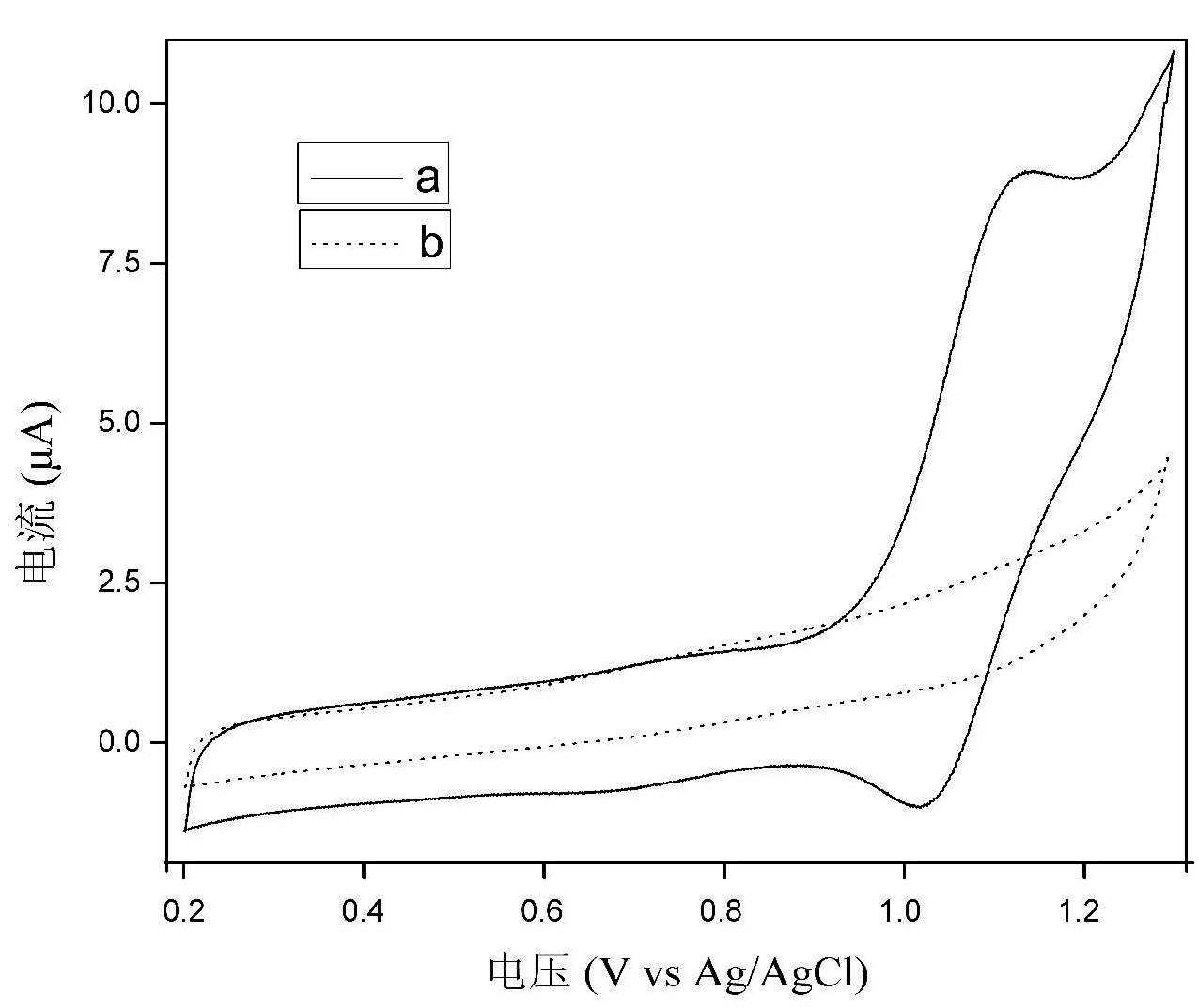
[0015] Electrochemical workstation and chemiluminescence analysis test system were used. 10 microliters of 2 g / L ruthenium terpyridine solution was dropped on the surface of the cleaned glassy carbon electrode; after natural drying, 10 microliters of 0.25 g / L graphene oxide solution was dropped on the surface of the electrode; after natural drying, 10 Microliter hydrazine solution (volume ratio water:hydrazine:ammonia=100:0.8:2) was dropped on the surface of the electrode as a reducing agent, and dried naturally at 25°C for 24 hours. Finally, the electrode was carefully washed with water to obtain a ruthenium-graphene film-modified ECL sensor. The sensor was scanned by cyclic voltammetry in a phosphate buffer solution containing tripropylamine (TPrA), and the electrochemiluminescence signal was used to quantitatively detect tripropylamine (TPrA), with a linear range of 5×10 -7 -2×10 -4 mol / L, the detection limit is 3×10 -8 mol / liter. By testing the ECL response of the sens...
Embodiment 2
[0017] Electrochemical workstation and chemiluminescence analysis test system were used. 10 microliters of 2 grams per liter of ruthenium terpyridine solution was dropped on the surface of the cleaned glassy carbon electrode; after natural drying, 10 microliters of 0.25 grams per liter of graphene oxide solution was dropped on the surface of the electrode; after natural drying again, 10 microliters of bovine serum albumin solution (50 g / L, pH=10) was dropped on the surface of the electrode as a reducing agent, and dried slowly at 30°C for 24 hours in a water bath. Finally, the electrode was carefully washed with water to obtain a ruthenium-graphene film-modified ECL sensor. The sensor was scanned by cyclic voltammetry in a phosphate buffer solution containing tripropylamine (TPrA), and the electrochemiluminescent signal was used to quantitatively detect tripropylamine (TPrA), with a linear range of 1×10 -6 -2×10 -4 mol / L, the detection limit is 5×10 -7 mol / liter. By testin...
Embodiment 3
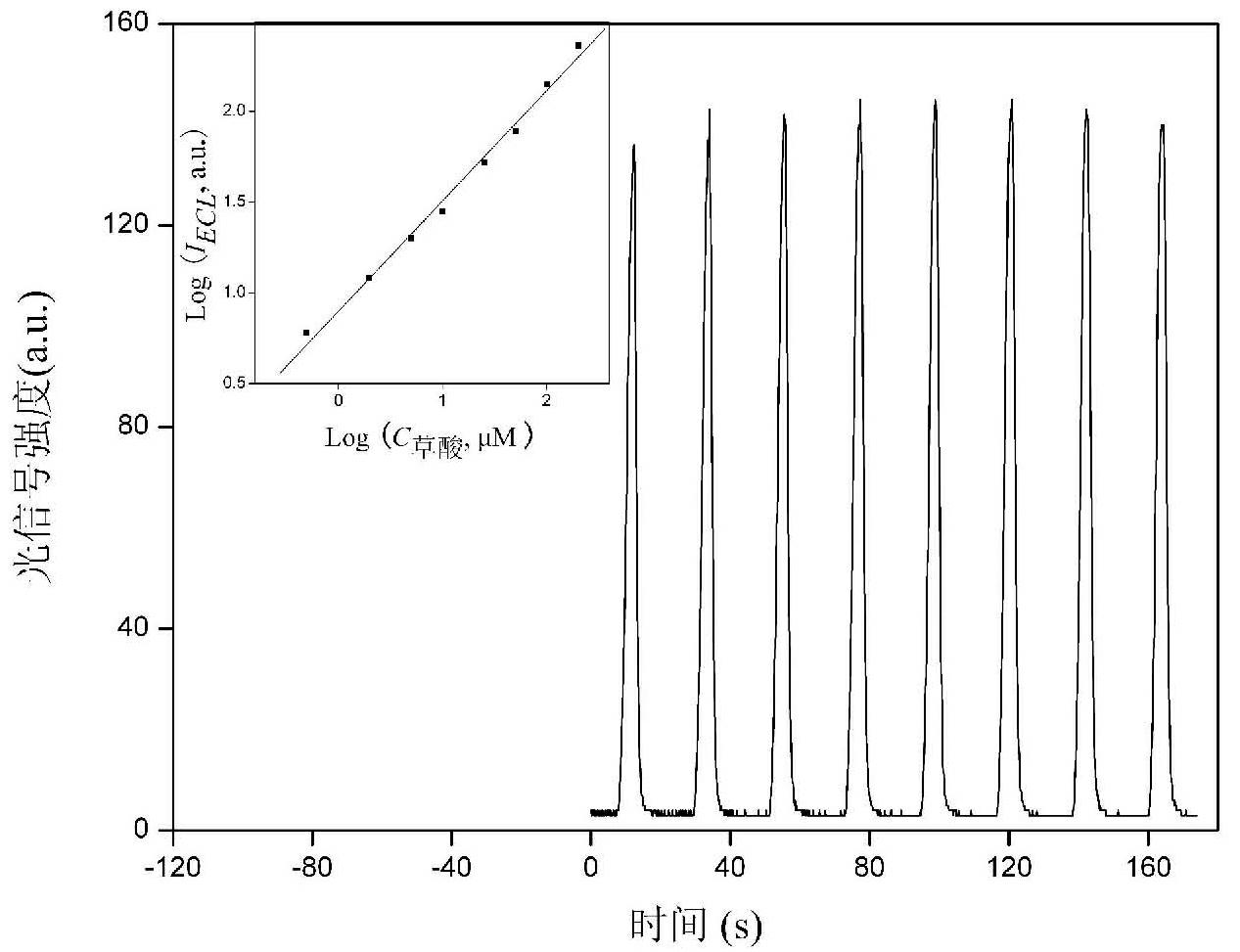
[0019] Electrochemical workstation and chemiluminescence analysis test system were used. 10 microliters of 2 g / L ruthenium terpyridine solution was dropped on the surface of the cleaned ITO electrode; after natural drying, 10 microliters of 0.25 g / L graphene oxide solution was dropped on the surface of the electrode; after natural drying again, 10 µl hydrogen bromide solution (5 x 10 -3 mol / L) as a reducing agent, was dropped on the surface of the electrode and dried naturally at 25°C for 24 hours. Finally, the electrode was carefully washed with water to obtain a ruthenium-graphene film-modified ECL sensor. The sensor was scanned by cyclic voltammetry in a phosphate buffer solution containing oxalic acid, and the electrochemiluminescent signal was used to quantitatively detect oxalic acid with a linear range of 1×10 -6 -2×10 -4 mol / L, the detection limit is 1×10 -7 mol / liter. By testing the ECL response of the sensor to oxalic acid every 5 days, it was found that 90% of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com