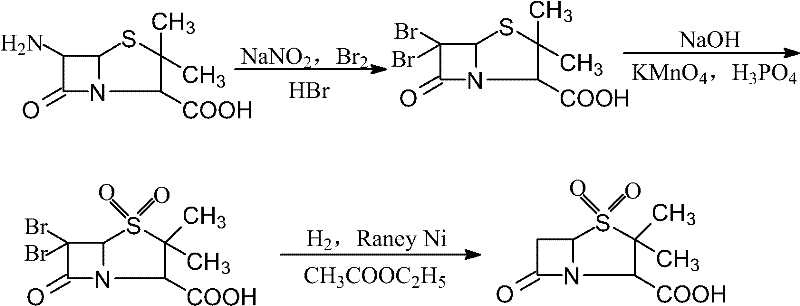
Synthesis method for sulbactam
A technology of sulbactamic acid and its synthetic method, which is applied in the field of sulbactamic acid synthesis, can solve the problems of unsuitability for large-scale industrial production, unstable quality of finished products, high cost of raw materials, etc., and achieve outstanding substantive characteristics, turbidity and Good purity and improved total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 500mL of ethyl acetate and 172.3g (1mol) of 47wt% hydrobromic acid into a 1000mL three-necked flask, stir and cool down to 0°C, then add 64g (0.4mol) of bromine and 6.9g (0.1mol) of sodium nitrite , and kept stirring, after dissolving, add 6-APA21.6g (0.1mol) in batches, control the reaction temperature at 4°C for 5h, then add 1mol.L -1 NaHSO 3 Neutralize the excess bromine until the reaction solution is milky yellow, let it stand and separate the layers, extract the aqueous layer twice with 100mL of ethyl acetate and combine the organic layer, then wash with 100mL of water, 100mL of 7% NaHCO 3 aqueous solution, saturated NaCl solution (50ml × 2) was washed until the organic layer was colorless, and the solvent was evaporated under reduced pressure to obtain 34.1 g of 6,6-dibromopenicillanic acid with a yield of 95% (according to 6-APA, the yield =Dry product weight / (6-APA substance quantity×359)).
[0019] (2) Dissolve 34.1g (0.095mol) of 6,6-dibromopenicilla...
Embodiment 2
[0022] (1) Add 600mL of ethyl acetate and 206.8g (1.2mol) of hydrobromic acid of 47wt% in a 1000mL three-necked flask, stir and cool down to 0°C, add 76.8g (0.48mol) of bromine and 8.28g (0.12mol) of sulfide Sodium nitrate, stirring continuously, add 25.9g (0.12mol) of 6-APA in batches after dissolving, control the reaction temperature at 4°C for 5h, then add dropwise 1mol·L -1 NaHSO 3 Neutralize the excess bromine until the reaction solution is milky yellow, let it stand and separate the layers, extract the aqueous layer twice with 100mL of ethyl acetate and combine the organic layer, then wash with 100mL of water, 100mL of 7% NaHCO 3 Aqueous solution, saturated NaCl solution (50ml * 2) wash until the organic layer is colorless, evaporate solvent under reduced pressure to obtain 6,6-dibromopenicillanic acid 41.2g, yield is 95.6% (according to 6-APA, yield =Dry product weight / (6-APA substance quantity×359)).
[0023] (2) Dissolve 41.2g (0.115mol) of 6,6-dibromopenicillanic a...
Embodiment 3
[0026] (1) Add 500mL of ethyl acetate and 172.3g (1mol) of 47wt% hydrobromic acid into a 1000mL three-necked flask, stir and cool down to 0°C, then add 64g (0.4mol) of bromine and 6.9g (0.1mol) of sodium nitrite , and kept stirring, after dissolving, add 21.6g (0.1mol) of 6-APA in batches, control the reaction temperature at 0°C for 3h, then add 1mol·L -l NaHSO 3 Neutralize the excess bromine until the reaction solution is milky yellow, let it stand and separate the layers, extract the aqueous layer twice with 100mL of ethyl acetate and combine the organic layer, then wash with 100mL of water, 100mL of 7% NaHCO 3 aqueous solution, saturated NaCl solution (50ml × 2) was washed until the organic layer was colorless, and the solvent was evaporated under reduced pressure to obtain 33g of 6,6-dibromopenicillanic acid with a yield of 92% (based on 6-APA, the yield = Dry product weight / (6-APA substance quantity×359)).
[0027] (2) Dissolve 33g (0.092mol) of 6,6-dibromopenicillanic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com