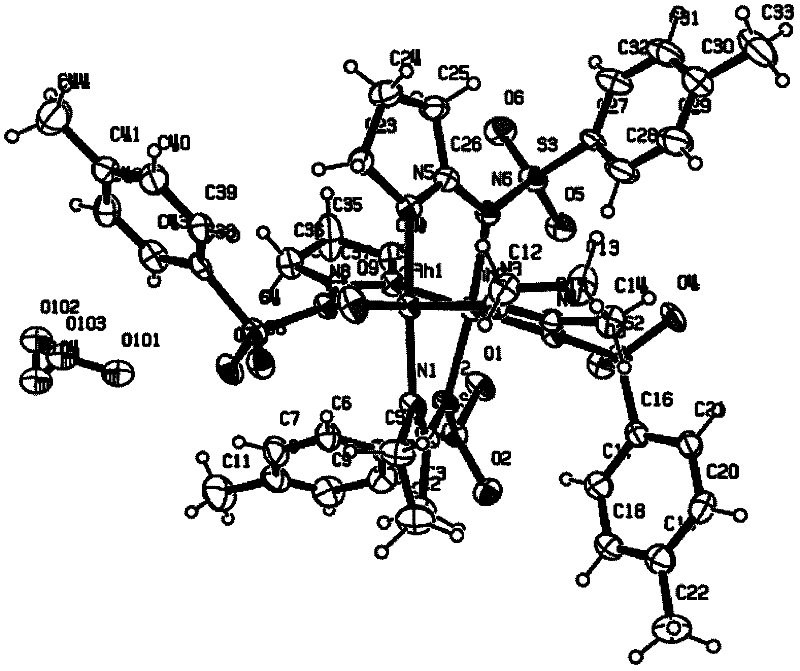
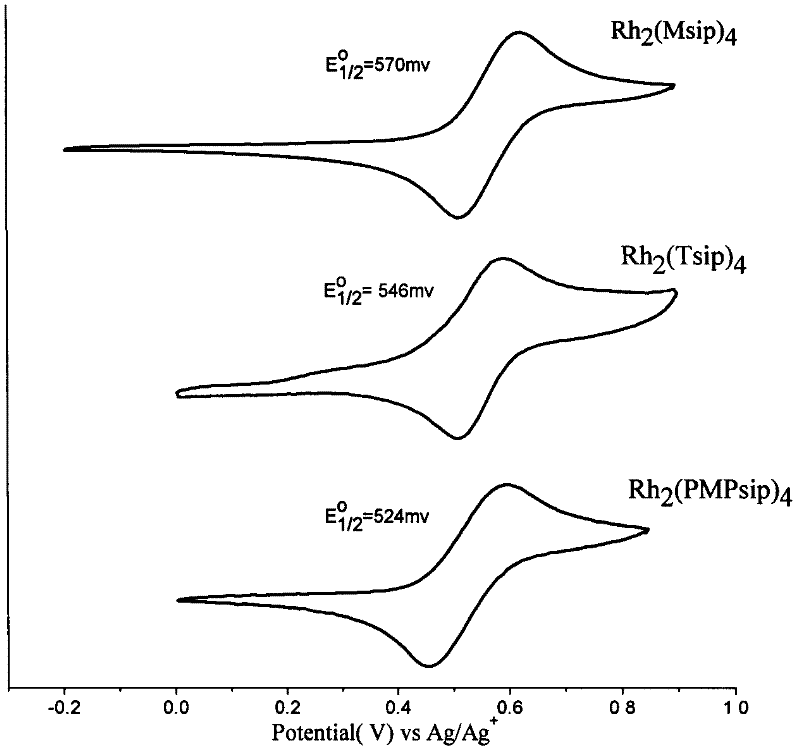
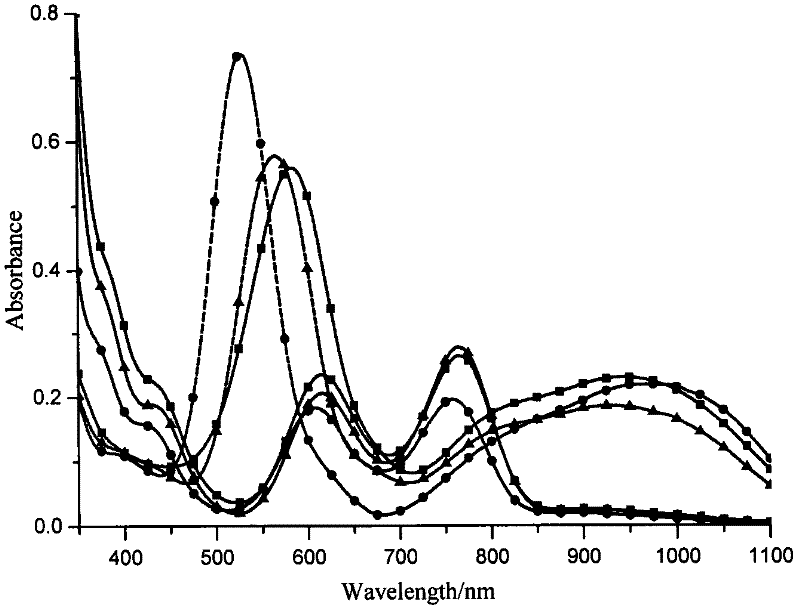
N,N-coordination dimeric rhodium (II) complex as well as preparation method and application thereof
A complex, dimerized rhodium technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemical methods, etc., to achieve the effect of good catalytic efficiency and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Ligand preparation:
[0054] Preparation of N-(2-pyrrolidinyl)-methylsulfonylimide
[0055] In a 25 mL two-necked flask, 2-methoxy-1-pyrpyrrolidine (0.99 g, 10.0 mmol), methylsulfonamide (0.71 g, 7.5 mmol) and isopropanol (15 mL) were added, and the mixture was stirred And reflux for 24 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from THF to obtain 1.05 g of white solid with a yield of 90%;
[0056] mp=144-145°C; 1 H NMR (400MHz, CDCl 3 )δ7.73(br s, 1H), 3.60(t, J=7.1Hz, 2H), 2.98(s, 3H), 2.69(t, J=7.6Hz, 2H), 2.13-2.05(m, 2H) ; 13 C NMR (100MHz, CDCl 3 )δ170.80, 46.62, 41.95, 33.23, 19.86. HRMS (ESI) C 5 h 10 N 2 NaO 2 S, measured value (calculated value) [(M+Na)+]: 185.0358 (185.0355);
[0057] Synthesis of Complex I:
[0058] Connect a Soxhlet extractor and a condenser to a 25mL two-necked round-bottom flask, add 4.0 g of a mixture of quartz sand and dry anhydrous sodium carbonate at a weight ratio of 1.5:3....
Embodiment 2
[0061] Ligand preparation:
[0062] Preparation of N-(2-pyrrolidinyl)-4-methylbenzenesulfonimide
[0063] In a 25 mL two-necked flask, 2-methoxy-1-pyrrolidine (0.99 g, 10.0 mmol), 4-methylbenzenesulfonamide (1.3 g, 7.5 mmol) and isopropanol (15 mL) were added, and the mixture Stirring and reflux reaction for 24 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 1.5 g of white solid with a yield of 86%;
[0064] mp=149-150°C; 1 H NMR (400MHz, CDCl 3 )δ8.00(br s, 1H), 7.81(d, J=8.2Hz, 2H), 7.28(d, J=8.2Hz, 2H), 3.60(t, J=7.2Hz, 2H), 2.69(t , J=8.1Hz, 2H), 2.41(s, 3H), 2.11-2.03(m, 2H); 13 C NMR (100MHz, CDCl 3 )δ171.51, 142.68, 139.50, 129.32, 126.40, 46.37, 32.90, 21.48, 20.01.HRMS(ESI)C 11 h 14 N 2 NaO 2 S measured value (calculated value) [(M+Na) + ]: 261.0675(261.0668);
[0065] Synthesis of Complex II:
[0066] Connect a Soxhlet extractor and a condenser to a 25mL two-necked round-bottom flas...
Embodiment 3
[0069] Ligand preparation:
[0070] Preparation of N-(2-pyrrolidinyl)-4-methoxybenzenesulfonimide
[0071] In a 25mL two-necked flask, add 2-methoxy-1-pyrrolidine (0.99g, 10.0mmol), 4-methoxybenzenesulfonamide (1.4g, 7.5mmol) and isopropanol (15mL), and The mixture was stirred and refluxed for 24 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 1.53 g of a white solid with a yield of 82%;
[0072] mp=146-147°C; 1 H NMR (400MHz, CDCl 3 )δ8.04(br s, 1H), 7.85(d, J=8.9Hz, 2H), 6.94(d, J=8.9Hz, 2H), 3.85(s, 3H), 3.59(t, J=7.1Hz , 2H), 2.69(t, J=8.0Hz, 2H), 2.13-1.99(m, 2H); 13 CNMR (100MHz, CDCl 3 )δ171.07, 162.66, 134.47, 128.65, 114.09, 55.76, 46.72, 33.34, 20.14. HRMS (ESI) C 11 h 14 N 2 NaO 3 S measured value (calculated value) [(M+Na) + ]: 277.0623(277.0617);
[0073] Synthesis of Complex III:
[0074] Connect a Soxhlet extractor and a condenser to a 25mL two-necked round-bottom flask, add 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com