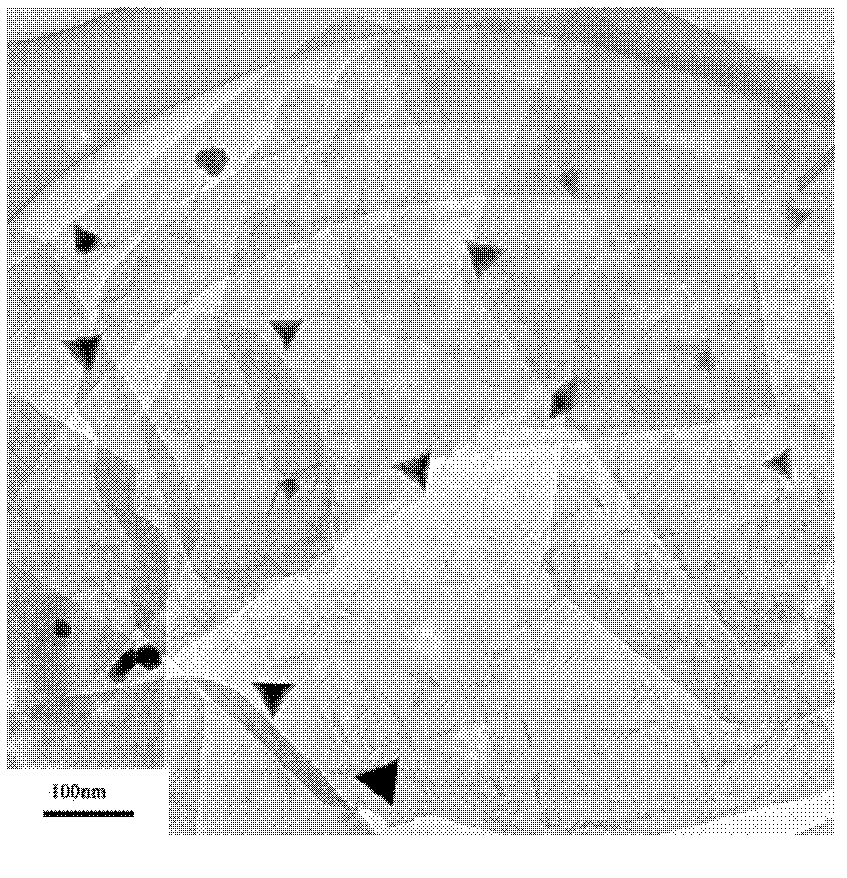Active component morphology controllable loaded noble metal catalyst and preparation method thereof
A technology of noble metal catalysts and active components, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. Low activity and selectivity, complex preparation methods and other problems, to achieve the effect of improving selectivity and activity, simple preparation method, and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A. Weigh 0.0207g NaCl and 0.0314g PdCl at a molar ratio of 2:1 2 Dissolved in 10ml of deionized water, prepared as metal Pd precursor Na 2 PdCl 4 solution;
[0036] B. Weigh 1.504gMg(NO 3 ) 2 ·6H 2 O, 1.097gAl(NO 3 ) 3 9H 2 O was dissolved in 80mL deionized water to prepare a metal salt solution, stirred until completely dissolved, then added the mixed solution prepared in step A, and stirred evenly;
[0037] C. Weigh 2.878g of hexamethylenetetramine (HMT), and dissolve it in the mixed solution prepared in step B under ice bath conditions;
[0038] D. Transfer the above mixed solution to a hydrothermal kettle, and conduct a crystallization reaction at 130° C. for 6 hours. The obtained product was washed and centrifuged until the supernatant had a pH value of 7, and the filter cake was dried in an oven at 70°C to obtain a Tetra-Pd-LDHs sample.
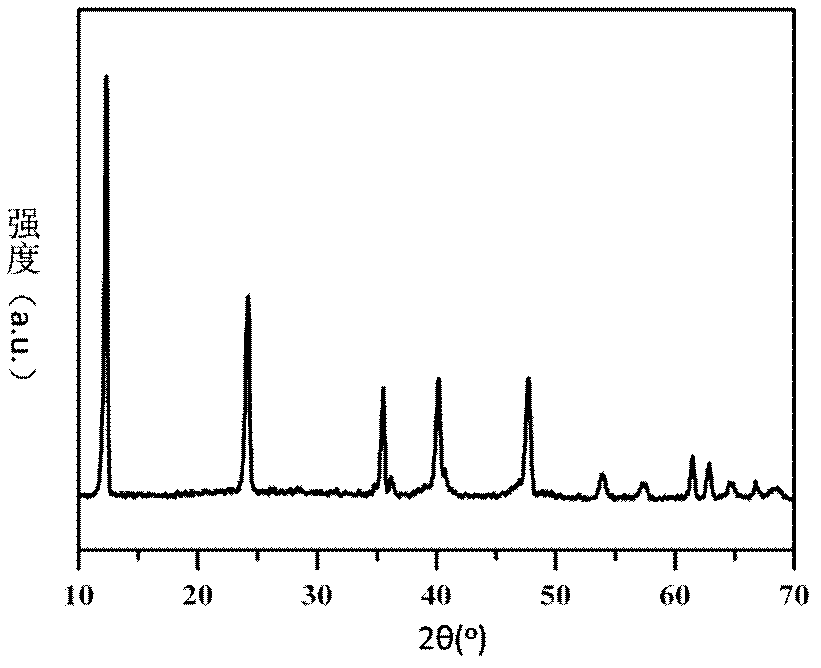
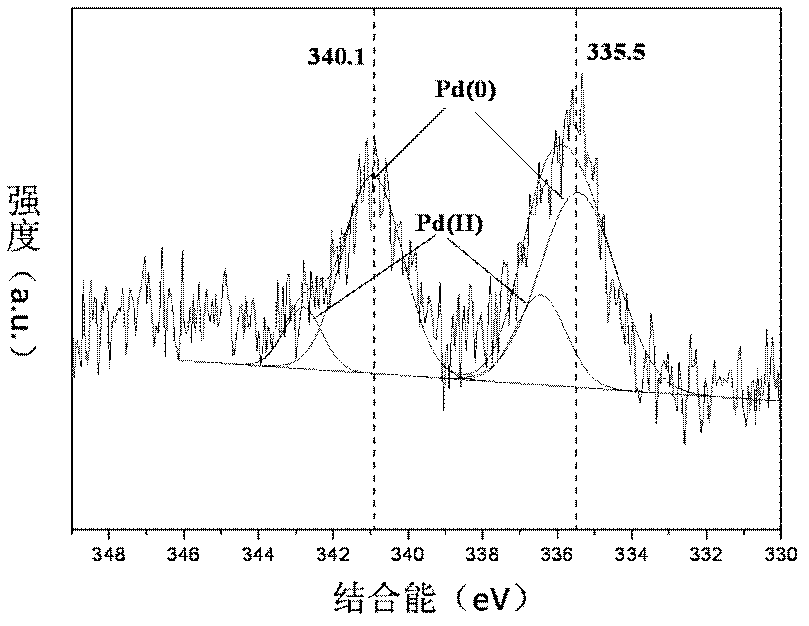
[0039] The XRD spectrogram, HRTEM photograph and XPS spectrogram of this catalyzer are respectively in the accompanyi...
Embodiment 2
[0041] A. Weigh 0.0217g NaCl and 0.0314g PdCl at a molar ratio of 2:1 2 Dissolved in 10ml of deionized water, prepared as metal Pd precursor Na 2 PdCl 4 solution;
[0042] B. Weigh 1.504gMg(NO 3 ) 2 ·6H 2 O, 1.097gAl(NO 3 ) 3 9H 2 O was dissolved in 80mL deionized water to prepare a metal salt solution, added the solution prepared in step A, and stirred evenly;
[0043] C. Add 1.300g of cetyltrimethylammonium bromide (CTAB) into solution B, stir until completely dissolved, then add 2.878g of hexamethylenetetramine (HMT), and stir in an ice bath. to obtain a homogeneous solution;
[0044] D. Transfer the above mixed solution to a hydrothermal kettle, and conduct a crystallization reaction at 130° C. for 6 hours. The obtained product was washed and centrifuged until the supernatant had a pH value of 7, and the filter cake was dried in an oven at 70°C to obtain the octa-Pd-LDHs sample.
Embodiment 3
[0046] Step A is with embodiment 1;
[0047] B. Weigh 0.705gMgSO 4 ·7H 2 O, 1.000gAl(SO 4 ) 2 16H 2 O was dissolved in 80mL deionized water to prepare a metal salt solution, added to the prepared solution in step A, and stirred evenly;
[0048] C, 0.177gPVP and 5ml concentration are that 1mmol / L KBr solution joins in solution B, stirs until fully dissolving, then adds 10ml dehydrated alcohol and 3.784g urea ((NH 2 ) 2 CO), fully stirred to obtain a homogeneous solution;
[0049]D. Transfer the above mixed solution to a hydrothermal kettle, and conduct a crystallization reaction at 130° C. for 6 hours. The obtained product was washed and centrifuged until the supernatant had a pH value of 7, and the filter cake was dried in an oven at 70°C to obtain a cubic-Pd-LDHs sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com