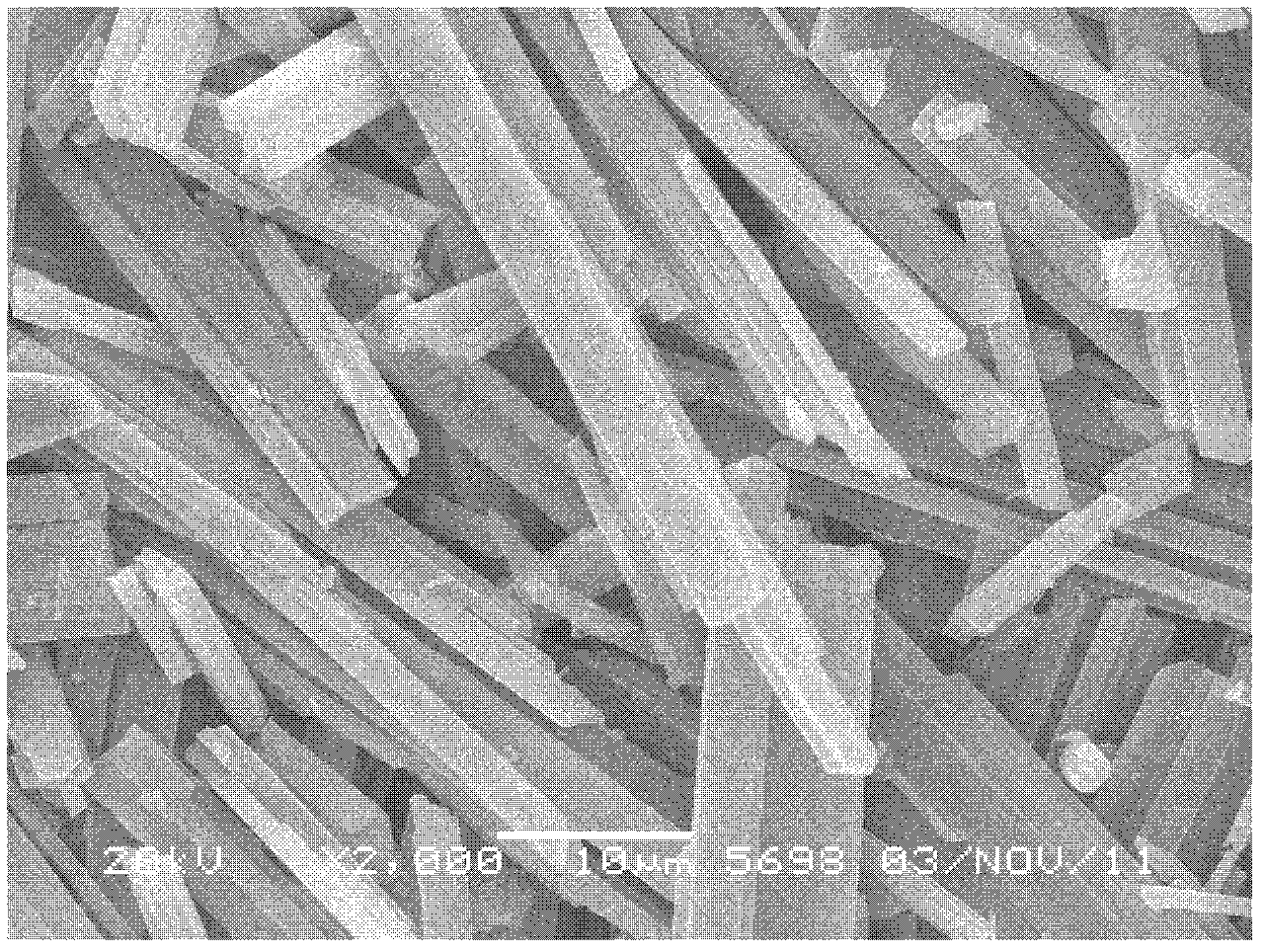Method for producing hydrofluoric acid from fluorite as raw material
A technology of hydrofluoric acid and raw materials, applied in the direction of fluorine/hydrogen fluoride, etc., can solve the problems of hindering the reaction, high sulfuric acid concentration, unable to complex calcium, etc., and achieve the effect of reducing leaching cost, simple leaching equipment, and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
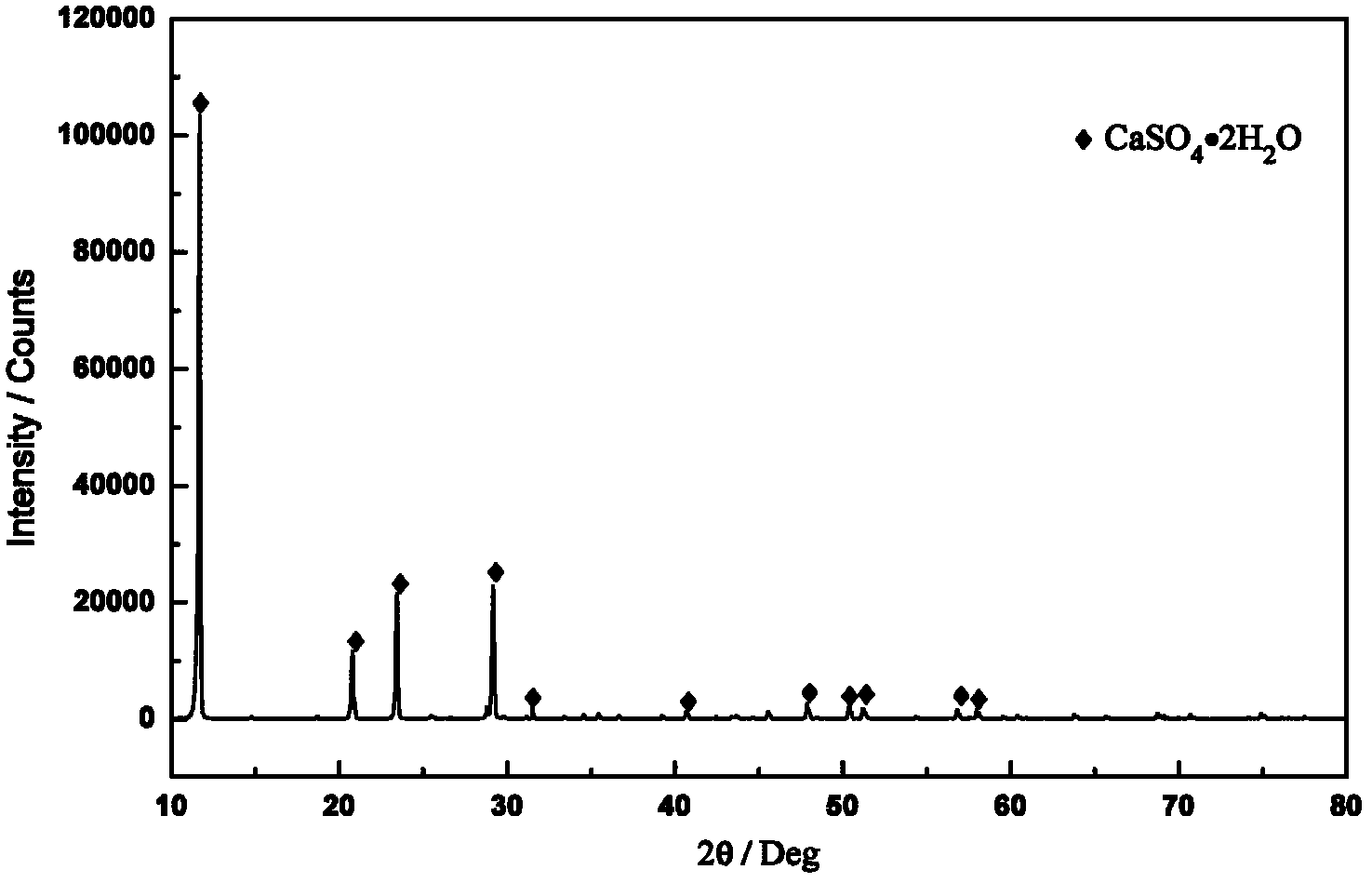
[0029] Fluorite ore (with CaF 2 97.2%) 1kg to prepare a mixed solution of phosphoric acid and sulfuric acid, P 2 O 5 The content is 25%, the concentration of sulfuric acid is controlled at 200g / L, the amount of dihydrate gypsum seed crystals added is 100g, the liquid-solid ratio is 10:1, the reaction temperature is 90°C, and the reaction time is 4h. The XRD pattern and SEM pattern of the obtained decomposition slag are as follows figure 1 and figure 2 shown. Under these conditions, CaF 2 The decomposition rate is 99.5%. After the reaction, filter, add the consumed sulfuric acid to the filtrate and return to a new round of leaching of fluorite ore powder. The HF gas generated during the reaction is evacuated and condensed, then absorbed and dehydrated by concentrated sulfuric acid, and further rectified to obtain hydrogen fluoride acid.
Embodiment 2
[0031] The filtrate that embodiment 2 produces is filled into sulfuric acid to decompose fluorite ore (containing CaF2) later to 200g / L 2 97.2%), dihydrate gypsum seed crystal addition 100g, liquid-solid ratio 10:1, reaction temperature 90 ℃, reaction time 4h, under this condition, CaF 2 The decomposition rate is 99.3%. After the reaction, filter, add the consumed sulfuric acid to the filtrate and return to a new round of leaching of fluorite ore powder. The HF gas generated during the reaction is evacuated and condensed, then absorbed and dehydrated by concentrated sulfuric acid, and further rectified to obtain hydrogen fluoride acid.
Embodiment 3
[0033] Fluorite ore (with CaF 2 97.2%) 1kg to prepare a mixed solution of phosphoric acid and sulfuric acid, P 2 O 5 The content is 35%, the concentration of sulfuric acid is controlled at 250g / L, the amount of dihydrate gypsum seed crystals added is 10g, the liquid-solid ratio is 5:1, the reaction temperature is 60°C, and the reaction time is 8h. Under these conditions, CaF 2 The decomposition rate is 98.4%. After the reaction, filter, add the consumed sulfuric acid to the filtrate and return to a new round of leaching of fluorite ore powder. The HF gas generated during the reaction is evacuated and condensed, then absorbed and dehydrated by concentrated sulfuric acid, and further rectified to obtain hydrogen fluoride acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com