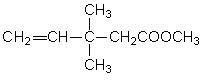
Method for continuously synthesizing 3,3-dimethyl-4-pentenoic acid methylester
A technology for the synthesis of methyl bentinate, which is applied in the field of continuous synthesis of methyl bentinate, can solve the problems of long reaction time, low product yield, and low reaction rate, and achieve shortening of the reaction cycle and the reduction of by-products The effect of low content and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A) Ingredients: Continuously add prenyl alcohol, trimethyl orthoacetate and phenol with a molar ratio of 1:3:0.012 to the batching kettle, and mix evenly to obtain a mixture;
[0022] B) Synthesis and separation 1: Continuously send the above-mentioned mixture to the first synthesis and separation tower for reaction, the feed temperature is controlled at 65°C, the temperature of the first synthesis and separation tower is controlled at about 80°C, The temperature is controlled at about 70°C, and the by-product methanol is continuously extracted;
[0023] C) Synthesis and separation 2: The materials in the bottom of the first synthesis and separation tower are continuously sent to the second synthesis and separation tower. At about 115°C, excess trimethyl orthoacetate is continuously distilled out;
[0024] D) Vacuum rectification: Continuously inject the material of the second synthesis separation tower into the rectification tank for vacuum distillation, the temperatu...
Embodiment 2
[0028] A) Ingredients: Continuously add isopentenol, trimethyl orthoacetate and phenol with a molar ratio of 1:3:0.005 to the batching kettle, and mix evenly to obtain a mixture;
[0029] B) Synthesis and separation 1: Continuously send the above-mentioned mixture to the first synthesis separation tower for reaction, the feed temperature is controlled at 62°C, the temperature of the first synthesis separation tower is controlled at about 80°C, the top The temperature is controlled at about 70°C, and the by-product methanol is continuously extracted;
[0030] C) Synthesis and separation 2: The materials in the bottom of the first synthesis and separation tower are continuously sent to the second synthesis and separation tower. At about 115°C, excess trimethyl orthoacetate is continuously distilled out;
[0031] D) Vacuum rectification: Continuously inject the material of the second synthesis separation tower into the rectification tank for vacuum distillation, the temperature ...
Embodiment 3
[0035] A) Ingredients: Continuously add prenyl alcohol, trimethyl orthoacetate and phenol in a molar ratio of 1:3:0.02 to the batching kettle, and mix evenly to obtain a mixture;
[0036] B) Synthesis and separation 1: Continuously send the above-mentioned mixture to the first synthesis separation tower for reaction, the feed temperature is controlled at 68°C, the temperature of the first synthesis separation tower is controlled at about 80°C, the top The temperature is controlled at about 70°C, and the by-product methanol is continuously extracted;
[0037] C) Synthesis and separation 2: The materials in the bottom of the first synthesis and separation tower are continuously sent to the second synthesis and separation tower. At about 115°C, excess trimethyl orthoacetate is continuously distilled out;
[0038] D) Vacuum rectification: Continuously inject the material of the second synthesis separation tower into the rectification tank for vacuum distillation, the temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com