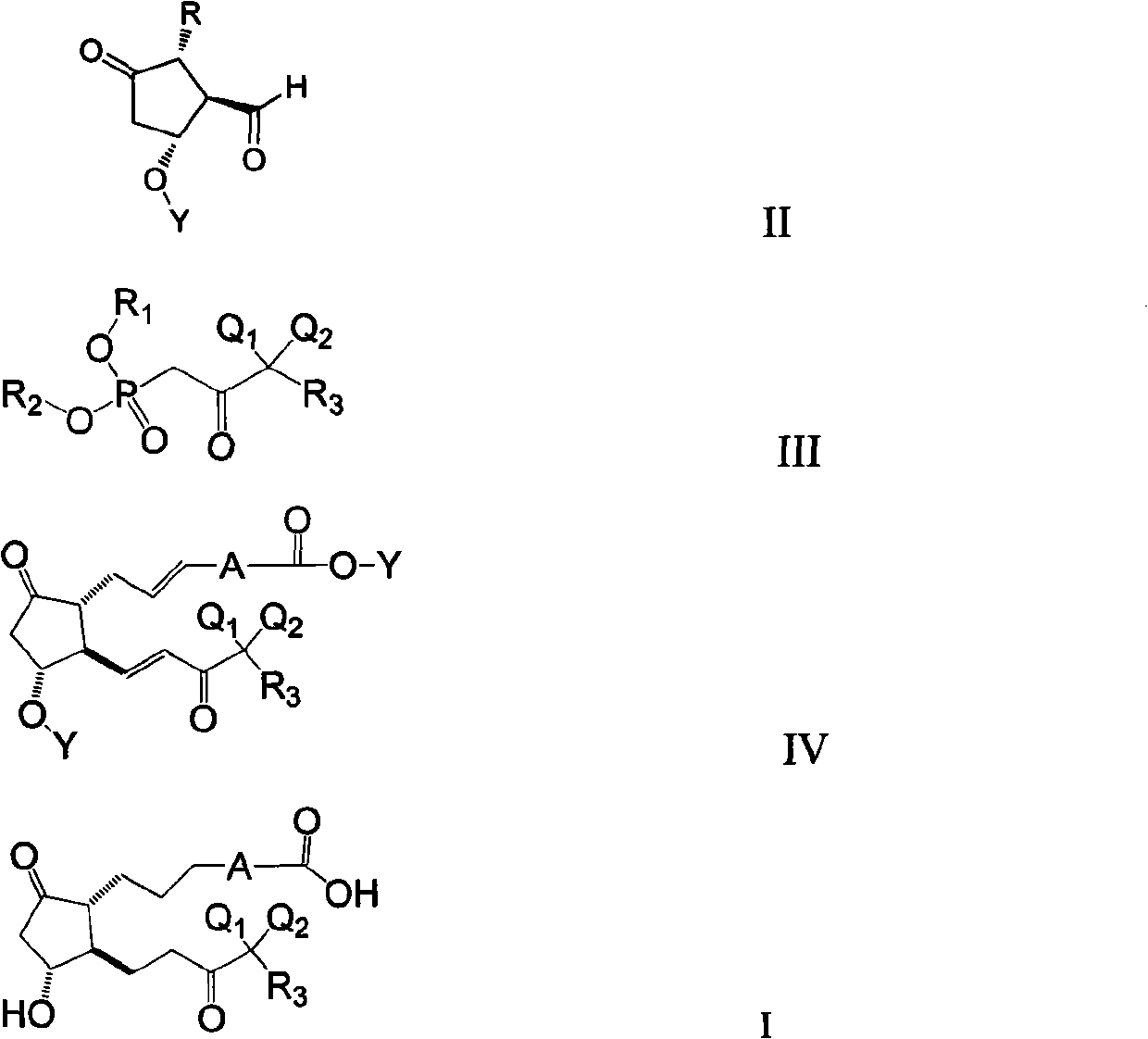
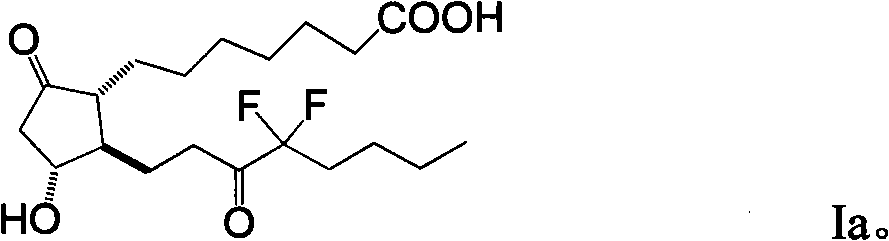
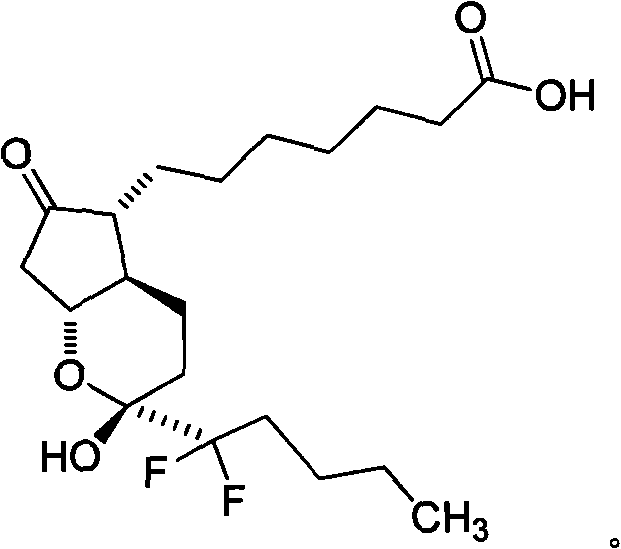
Preparation method of prostaglandin derivative
A technology for preparation steps and compounds, which is applied in the field of preparation of prostaglandin derivatives, can solve problems such as high requirements for reaction equipment and threats to production safety, and achieve the effects of simplifying reaction conditions, reducing requirements, and reducing pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] In a 250mL dry three-necked flask, add 3 (4.06g, 1eq.) and 50mL of anhydrous toluene, lower the temperature to -70°C under the protection of nitrogen, add 34mL DIBAL-H (2.0eq., 1M) dropwise, and drop it for 1.5 hours. . And stirred at this temperature for 1 hour, TLC detected that the reaction was completed, 2 g of isopropanol (4 eq.) was added dropwise to quench the reaction, the temperature was raised to room temperature, and stirring was continued for 1 hour. 40 mL of saturated sodium potassium tartrate solution and 40 mL of diethyl ether were added dropwise, stirred for 5 minutes, allowed to stand to separate layers, and the organic phase was separated, dried over anhydrous sodium sulfate, and concentrated to obtain 4.08 g of crude compound 5. It was directly used for the next reaction without purification.
Embodiment 2
[0047]
[0048] Add compound 4 (15g, 2.5eq.) and 50mL of anhydrous THF into a 250mL three-neck flask, stir in an ice bath, slowly add LiHMDS (66.5mL, 1M) THF solution dropwise to obtain a brick-red solution, stir at 20°C for 1 hour . 5g (1.0eq.) of compound 5 was added to the reaction solution, and stirred at 20°C for 3 hours. TLC detected the end of the reaction, quenched the reaction with 40 mL of ice water, concentrated under reduced pressure to remove THF, extracted the aqueous phase with methyl tert-butyl ether (50 mL×3), washed once with 100 mL of saturated sodium chloride, dried over anhydrous sodium sulfate, and Concentration gave 6.1 g of crude compound 6. Purification by column chromatography yielded 4.6 g of compound 6.
Embodiment 3
[0050]
[0051] Add 10 mL of acetonitrile to compound 6 (4.6 g), stir to dissolve, add diisopropylethylamine (2.66 mL), 4-methoxybenzyl bromide (1.8 mL), and stir at room temperature for 16 hours. The completion of the reaction was detected by TLC, and the reaction solution was concentrated under reduced pressure and purified by column to obtain compound 7 (4.9 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com