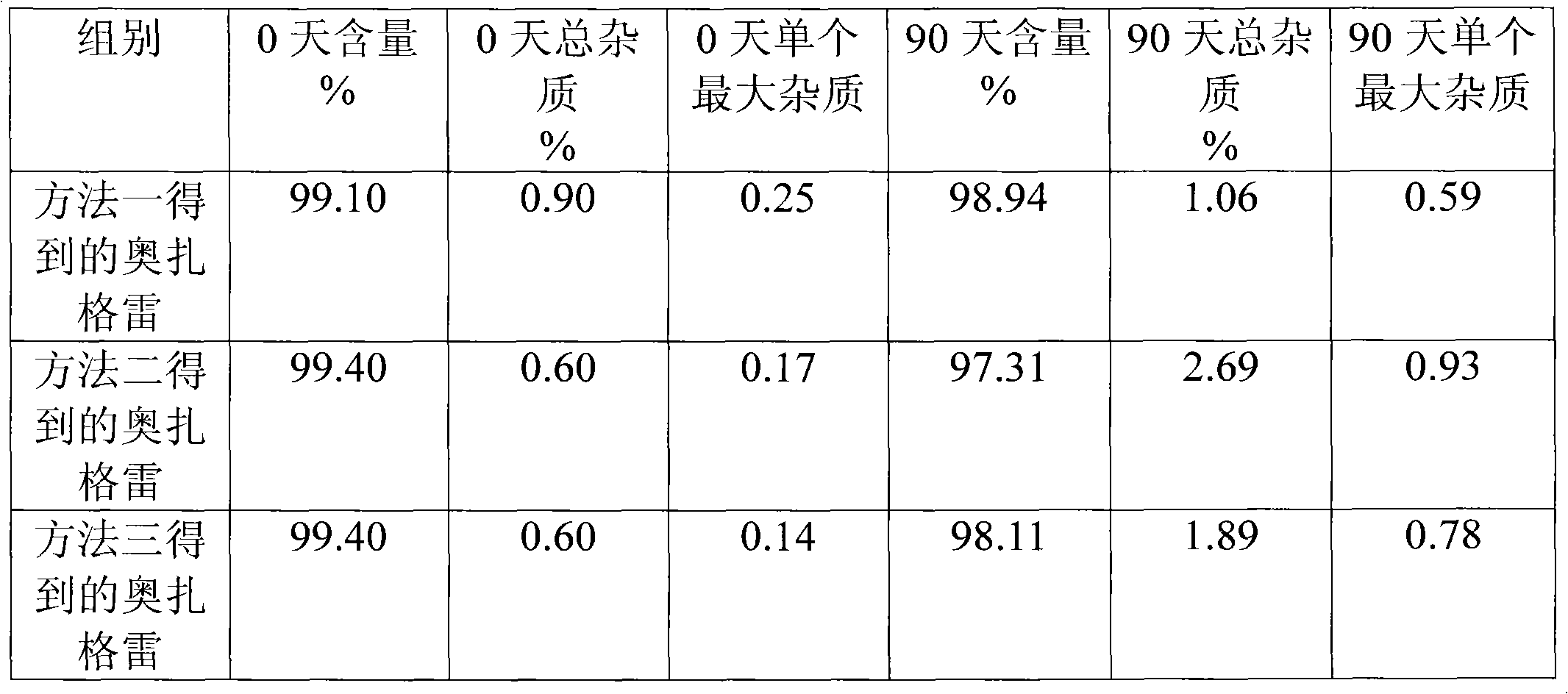
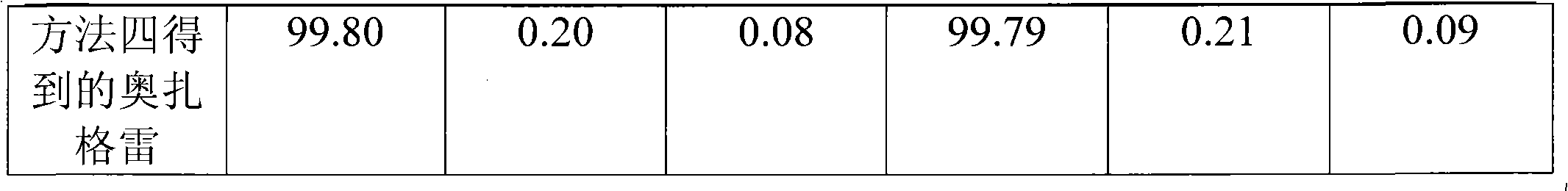
Synthetic method of ozagrel
A technology of ethyl rathate and ethyl cinnamate, applied in the field of medicine, can solve problems such as low yield of the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
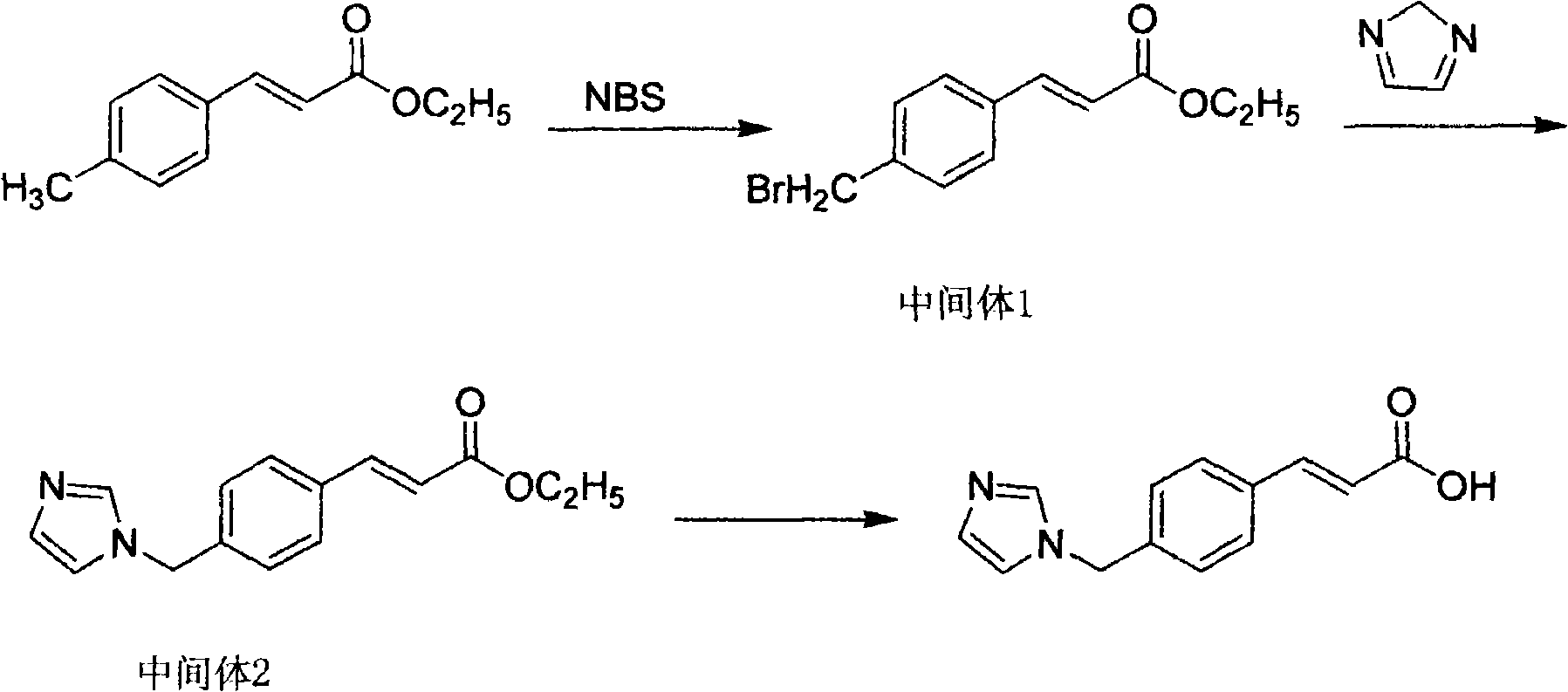
[0064] Take 380g of ethyl p-methylcinnamate, 708g of NBS, 6.56g of azobisisobutyronitrile, and 1000ml of acetonitrile, heat to reflux for 5h, filter, and concentrate the filtrate to obtain an oil, which is recrystallized with 800ml of 95% ethanol and dried. Obtained 461.3 g of ethyl p-bromomethyl cinnamate with a melting point of 43-45°C. Yield 85.5%.
[0065] Get 461.3g of ethyl p-bromomethyl cinnamate, 122g of imidazole, 373g of anhydrous potassium carbonate, 3.0g of potassium iodide and 1450ml of acetone and mix completely, the mixture is heated to reflux for 15 hours, filtered, and the filtrate is concentrated to recycle the organic solvent to the utmost, and the residue Dissolve in 2600ml of ethyl acetate, wash with water 3 times, 900ml each time, dry the organic phase with MgSO4, filter, concentrate the filtrate until the organic solvent is exhausted, dry to obtain a solid, and use a volume ratio of 1:1 ethyl acetate-petroleum ether Recrystallized, concentrated, and dri...
Embodiment 2
[0068] Take 1900g of ethyl p-methyl cinnamate, 3540g of NBS, 32.8g of azobisisobutyronitrile, and 5L of acetonitrile, heat to reflux for 6h, filter, and concentrate the filtrate to obtain an oil, which is recrystallized with 5L of 95% ethanol and dried. 2290 g of ethyl p-bromomethyl cinnamate was obtained, with a melting point of 43-45°C. Yield 85.1%.
[0069] Get 2290g of ethyl p-bromomethyl cinnamate, 609.3g of imidazole, 1855g of anhydrous potassium carbonate, 14.9g of potassium iodide and 7.2L of acetone and mix completely, the mixture is heated to reflux for 16 hours, filtered, and the filtrate is concentrated to recycle the organic solvent to the utmost, and the remaining The product was dissolved in 13.5L of ethyl acetate, washed with water 3 times, 4L each time, the organic phase was dried with MgSO4, filtered, the filtrate was concentrated to the organic solvent, dried to obtain a solid, and the volume ratio was 1: 1 ethyl acetate- Petroleum ether was recrystallized,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com