Method for making color flame candle
A color flame candle and technology for manufacturing method, which are applied to candle preparation devices and other directions, can solve problems such as harmfulness, large covering effect, and difficulty in forming, and achieve the effects of high color purity, reduced loss, and difficulty in bending.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of embodiment 1 green flame candle
[0035] 1. Put an appropriate amount of cotton thread in 6% sodium hydroxide solution, heat and reflux for 2 hours, take it out and rinse it repeatedly with deionized water, after drying, add the cotton thread to a mixed solution with a concentration of 2% hydrochloric acid and 3% ammonium chloride , heated to reflux for 2 hours, took out, rinsed repeatedly with deionized water, and dried.
[0036] 2. Take part of the above-mentioned treated cotton thread and soak it in 0.5% nitric acid solution for half an hour, take it out and dry it to form a burning core.
[0037] 3. Take part of the cotton thread treated in step 1 and put it in 4% boric acid solution, take it out after soaking for 1 hour, fully dry it, put it in 3% ammonium nitrate solution for half an hour, take it out and dry it to get the color core.
[0038] 4. Wind a hair color wick and a burning wick to form a candle wick.
[0039] 5. Weigh 6.500g o...
Embodiment 2
[0041] The preparation method of embodiment 2 blue flame candle
[0042] Steps 1 and 2 are the same as the above example.
[0043] Part of the candle wick treated in step 1 is soaked in 15% copper chloride solution for one hour, taken out, dried, soaked in 3% ammonium nitrate solution for half an hour, taken out and dried to form the color core.
[0044] Wind a hair wick with a burner wick to form a candle wick.
[0045] Take by weighing 7.200g trimethyl citrate, 0.950g stearic acid amide, 0.190g stearyl alcohol, add 1.100g anhydrous cupric chloride after being placed in container heating and melting, make cupric chloride be evenly dispersed in the molten liquid, Put the candle wick in the mold, pour the molten candle liquid, and cool it to form a blue flame candle.
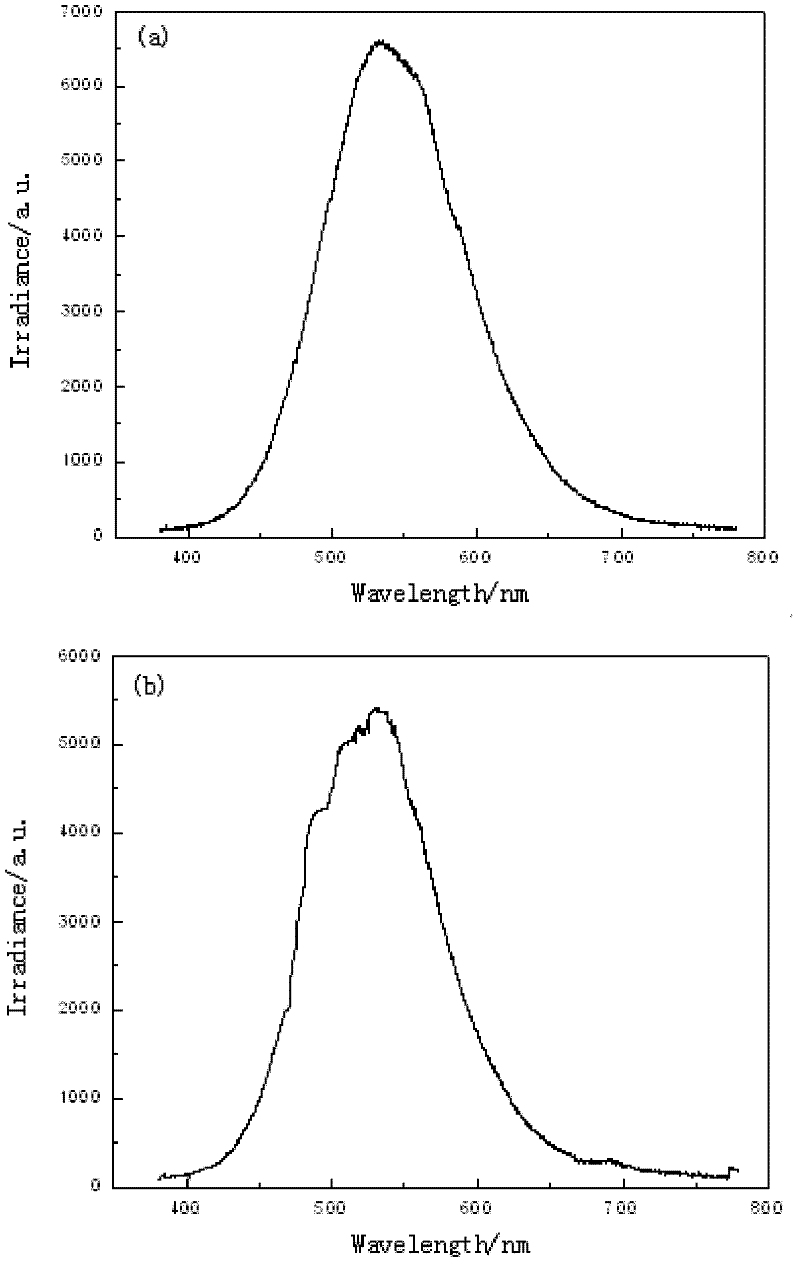
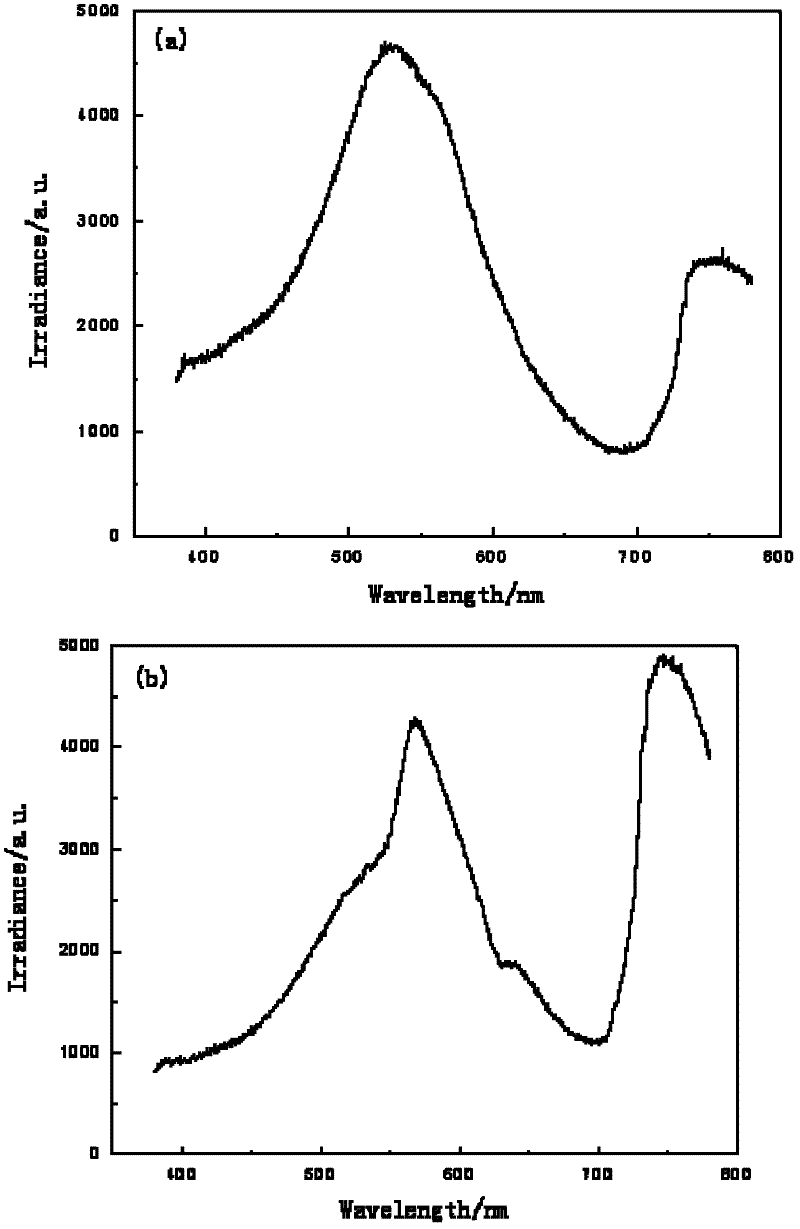
[0046] Detection method is with embodiment 1, figure 2 The blue flame of the candle in Example 2 is relatively pure, with less variegation, and the commercially available blue candle flame has more variegated...
Embodiment 3
[0047] The preparation method of embodiment 3 red flame candle
[0048] Steps 1 and 2 are the same as the above example.
[0049] Part of the candle wick treated in step 1 is soaked in 20% lithium chloride solution for one hour, taken out, dried, soaked in 3% ammonium nitrate solution for half an hour, taken out and dried to form a color core.
[0050] Wind a hair wick with a burner wick to form a candle wick.
[0051] Weigh 7.300g of trimethyl citrate, 0.790g of stearic acid amide, 0.200g of stearyl alcohol, and 1.100g of anhydrous lithium acetate, heat and melt in a container, place the candle wick in a mold, and pour the molten candle liquid , cooled and shaped into a red flame candle.
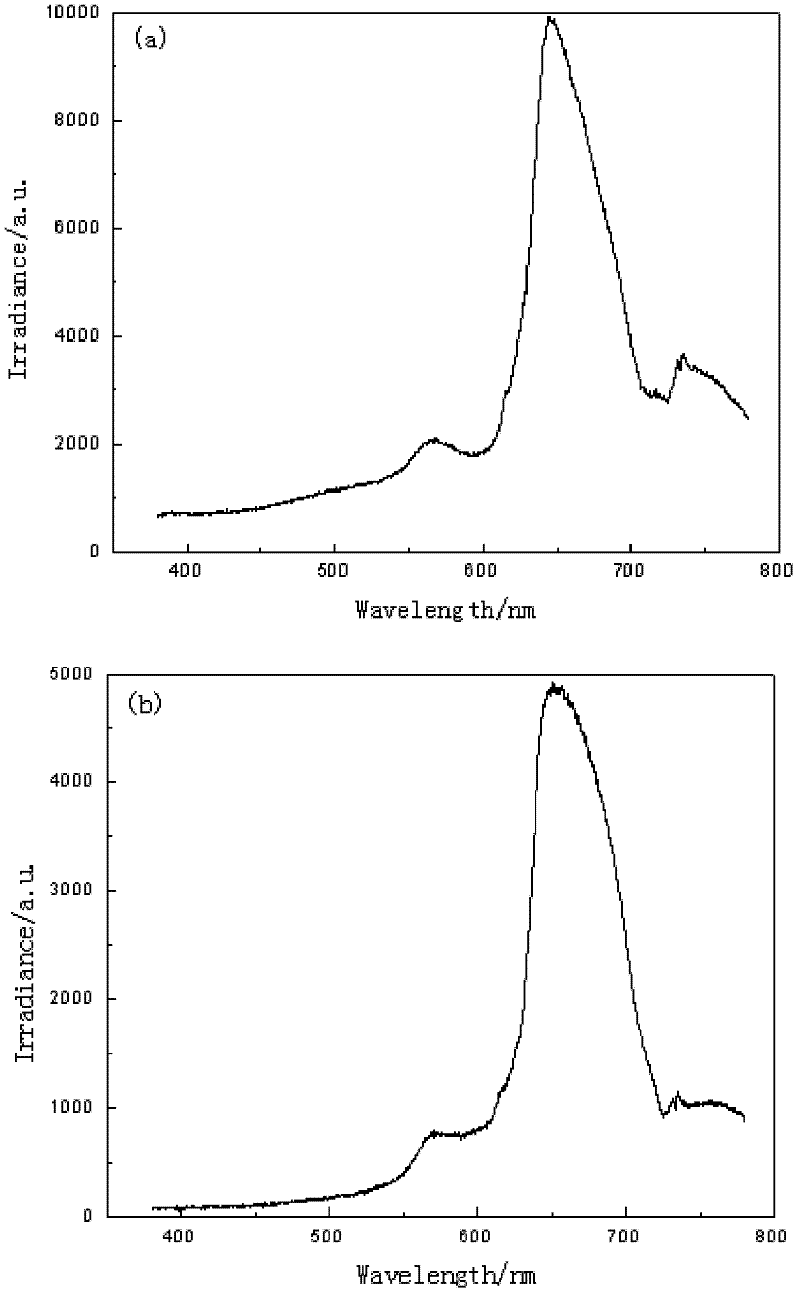
[0052] Detection method is with embodiment 1, image 3 The light intensity of the red flame candle in Example 3 is much higher than that of the commercially available red flame candle, and compared with the commercially available candles, the wavelength range of the flame in Example 3 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com