Pichia pastoris system capable of co-expressing An Man5A and XynII
A Pichia pastoris, expression system technology, applied in the field of bioengineering, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Construction, expression and product activity determination of engineering bacteria GS115 / man5A
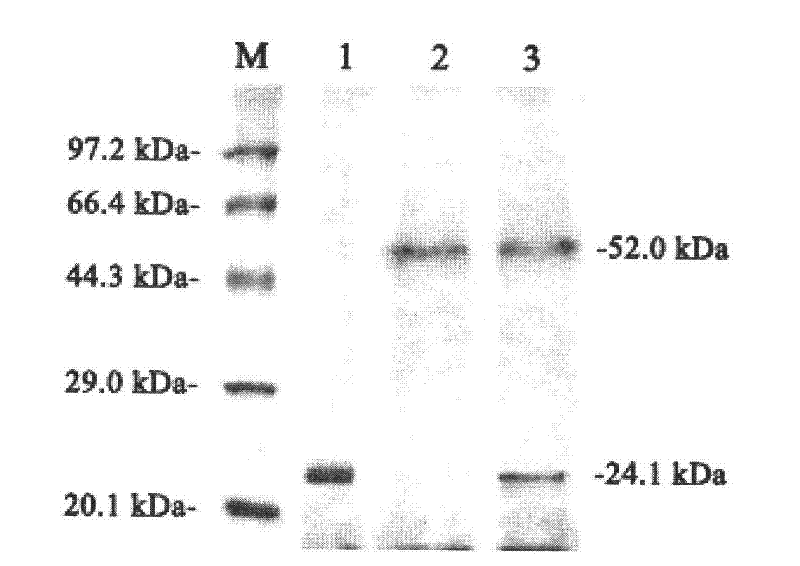
[0016] Linearize pPICZαA-man5A with Sac I, perform electrotransformation of GS115 competent cells according to the Pichia expression manual, and use Zeocin to screen to obtain high-copy Pichia recombinant GS115 / man5A. The engineered bacterium was induced with 1.0% methanol for 96 hours, and the recombinant mannanase activity in the fermentation broth was measured by DNS method up to 29.05IU / mL. SDS-PAGE electrophoresis showed that the molecular weight of the recombinant mannanase was 52.0kDa.
Embodiment 2
[0017] Example 2 Construction, expression and product activity determination of engineering bacteria GS115 / man5A-xynII
[0018] Linearize pPIC9k-xynII with Sal I, perform electrotransformation of GS115 / man5A competent cells according to the Pichia expression manual, and use G418 to obtain high-copy Pichia recombinant GS115 / man5A-xynII. The engineering bacterium was induced with 1.0% methanol for 96 hours. The activity of recombinant xylanase in the fermentation broth was 112.10IU / mL and the activity of recombinant mannanase was still 29.05IU / mL as measured by DNS method. SDS-PAGE electrophoresis showed that the molecular weights of recombinant xylanase and mannanase were 24.1kDa and 52.0kDa, respectively, as figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com