Heterocycle polymer alkaline anion-exchange membrane and method for producing same
A basic anion and nitrogen heterocyclic compound technology, applied in the field of polymer ion exchange membranes, can solve the problems of mechanical property degradation, high swelling rate, quaternary ammonium group falling off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
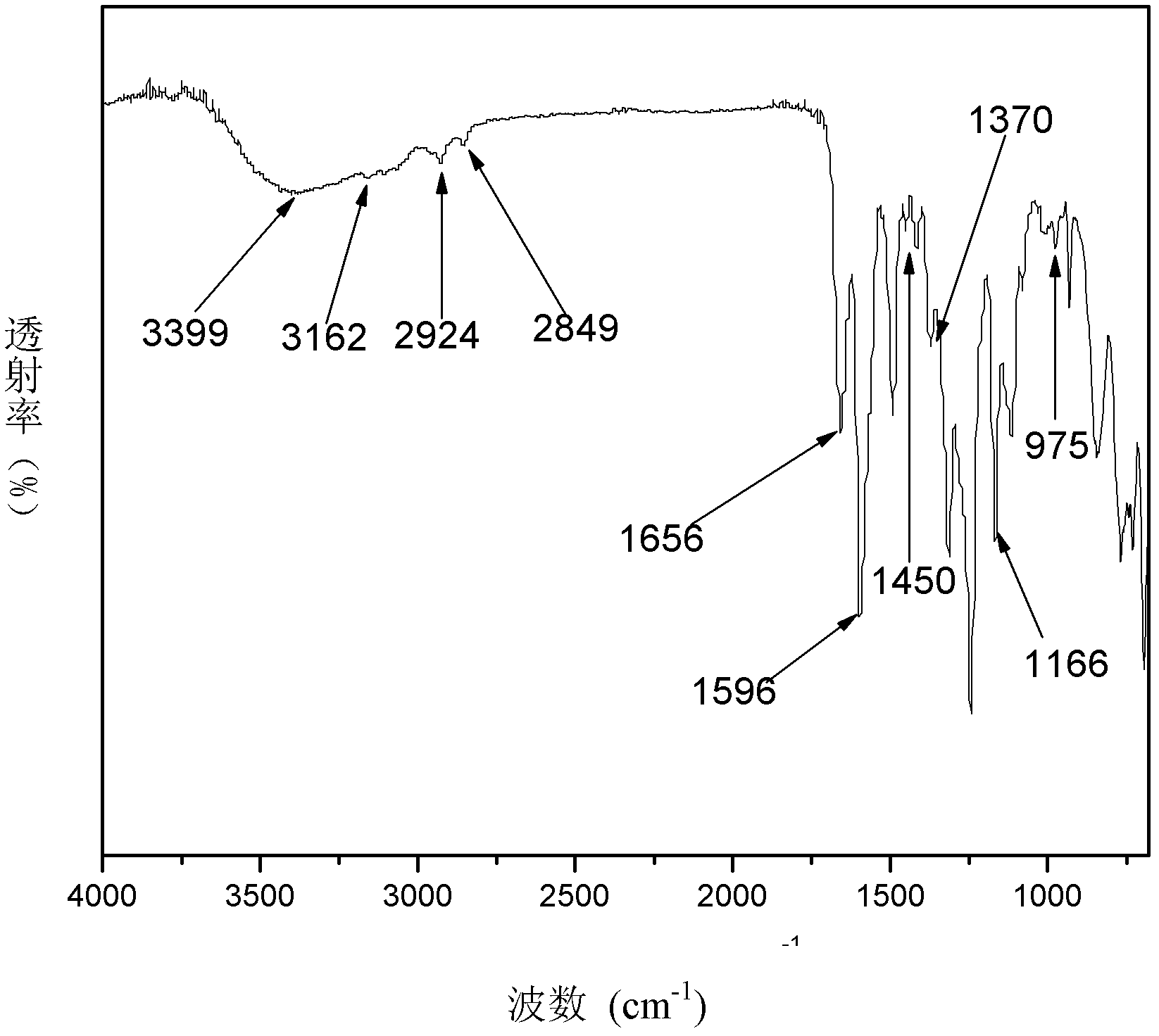
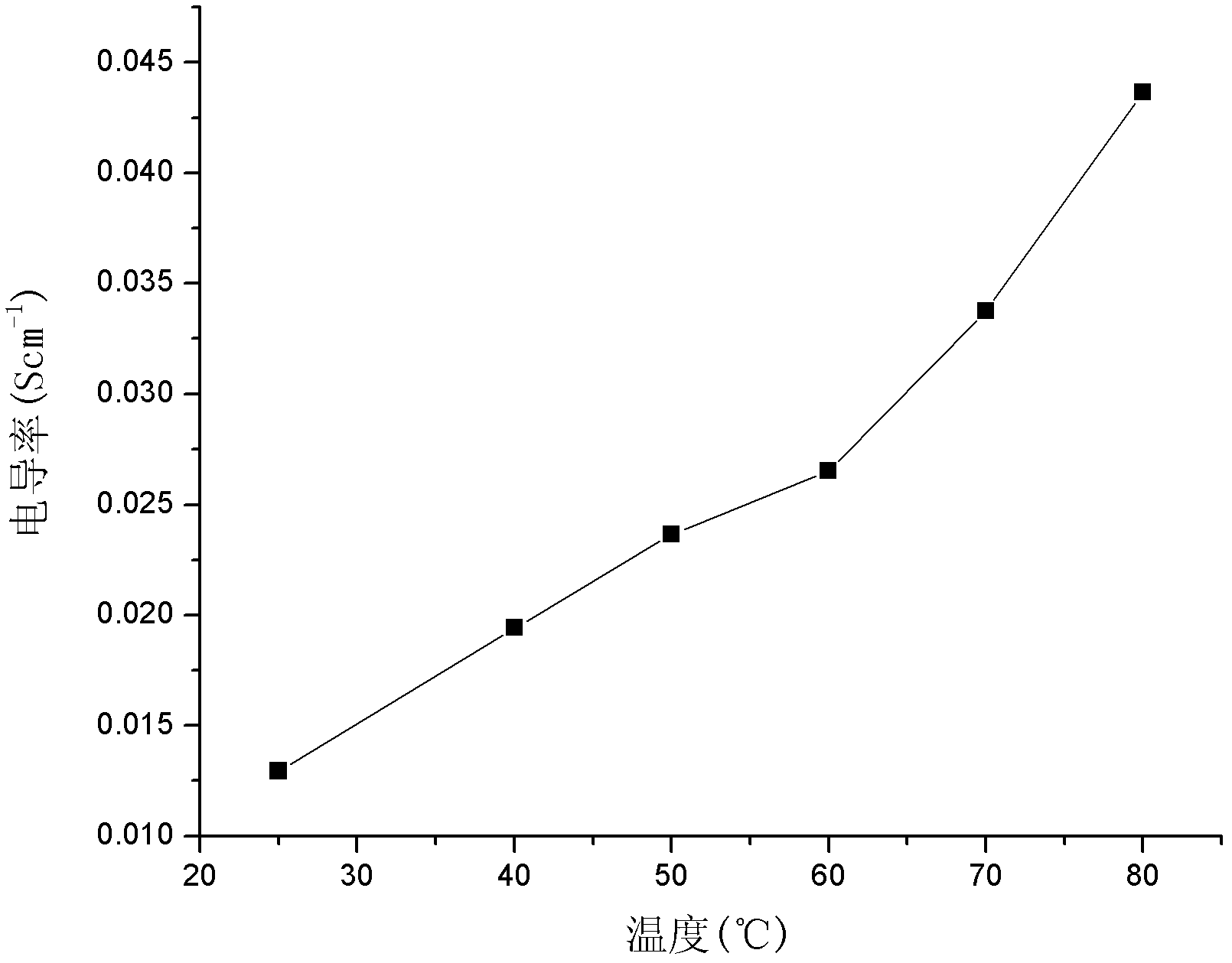
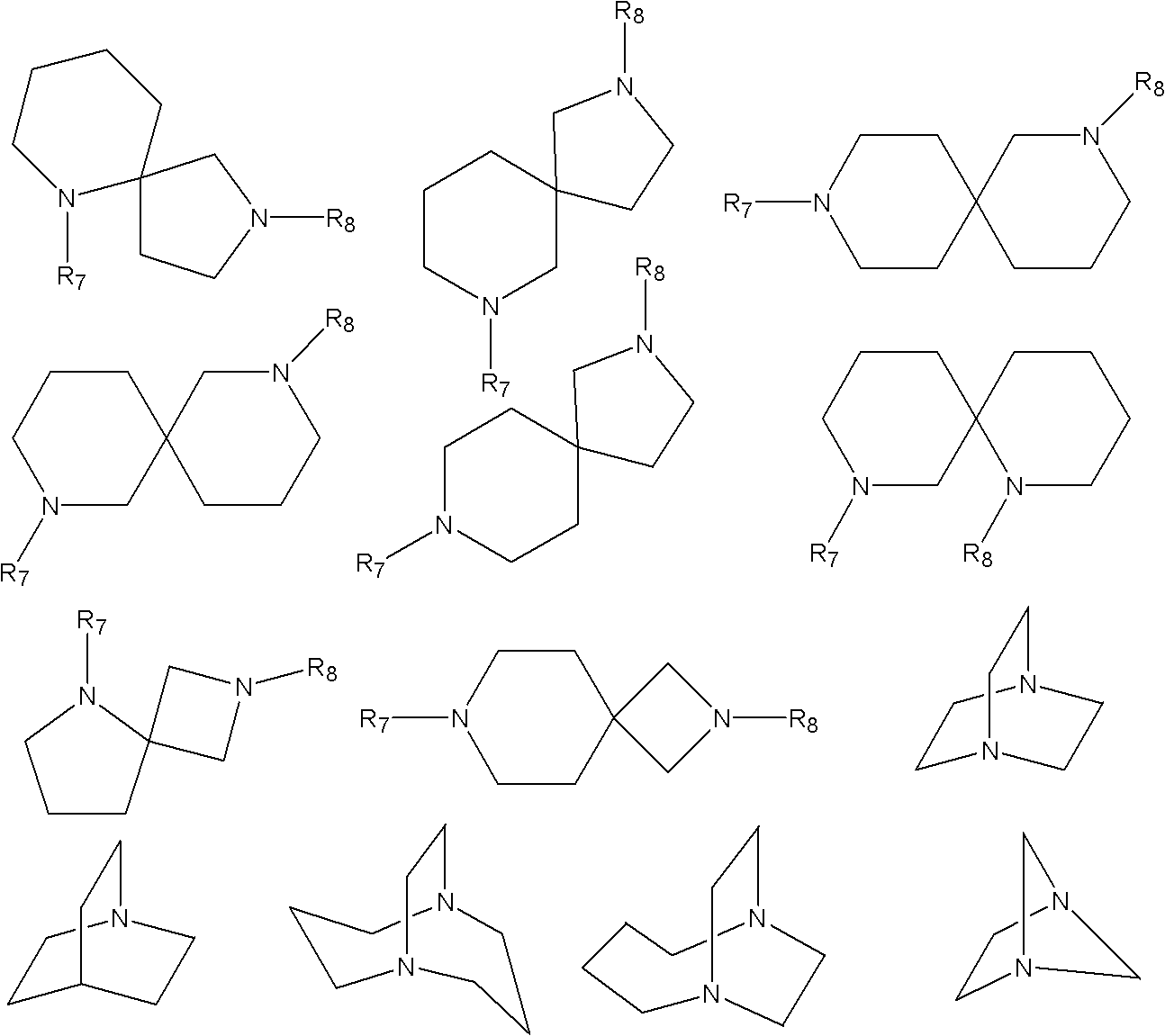
[0072] Dissolve 100g of polyetheretherketone (PEEK) in 650ml of concentrated sulfuric acid, add dropwise 20ml of chloromethyl octyl ether after dissolving, and stir at room temperature for 3 hours. After the reaction, pour the reactant into the ice-water mixture to precipitate the product. The product was washed with water until neutral, filtered, and dried to obtain chloromethylated polyetheretherketone (CMPEEK). Weigh 10g of CMPEEK and dissolve it in 6.5ml of N,N-dimethylformamide (DMF), add 0.20g of pyrazole, stir and react for 10min to obtain a casting solution. Membranes were prepared by solution casting, dried in an oven at 60°C, then soaked in 1mol / L KOH solution for 24 hours, taken out, and fully rinsed with deionized water to obtain basic anion exchange membranes.
[0073] In this embodiment, such as using phthalazinone biphenyl polyether ketone, polyether sulfone, polyether sulfone ketone, polyether ketone ketone, polyether nitrile ketone, polyether nitrile sulfone k...
Embodiment 2
[0075]Dissolve 100g of phthalazinone biphenyl polyether ketone ketone (PPEKK) in 800ml of concentrated sulfuric acid, add dropwise 20ml of chloromethyl butyl ether after dissolving, stir and react at 45°C for 3h, and pour the reactant into ice-water mixture after the reaction , the product precipitated out. The product was washed with water until neutral, filtered and dried to obtain chloromethylated naphthalene polyether ketone ketone (CMPPEKK). Weigh 10g of CMPPEKK and dissolve it in 6ml of N,N-dimethylformamide, add 0.20g of 1-methylimidazole, stir and react for 10min to obtain a casting solution. Membranes were prepared by solution casting, dried in an oven at 60°C, then soaked in 1mol / L KOH solution for 24 hours, taken out, and fully rinsed with deionized water to obtain basic anion exchange membranes.
[0076] In this embodiment, such as using phthalazinone biphenyl polyether ketone, polyether sulfone, polyether sulfone ketone, polyether nitrile ketone, polyether nitril...
Embodiment 3
[0078] Dissolve 100g of polysulfone (PSF) in 800ml of concentrated sulfuric acid, add 20ml of chloromethyl butyl ether dropwise after dissolving, stir at room temperature for 3h, and pour the reactant into ice-water mixture after the reaction to precipitate the product. The product was washed with water until neutral, filtered, and dried to obtain chloromethylated polysulfone (CMPSF). Weigh 10g of CMPSF and dissolve it in 8ml of N,N-dimethylacetamide, add 0.20g of imidazole, stir and react for 10min to obtain a casting solution. The film was prepared by solution casting method, adding 0.10g React for 1 hour, dry in an oven at 60°C, then soak the membrane in 1mol / L KOH solution for 24 hours, take it out, and rinse it with deionized water to obtain a basic anion exchange membrane.
[0079] In this embodiment, such as using phthalazinone biphenyl polyether ketone, polyether sulfone, polyether sulfone ketone, polyether ketone ketone, polyether nitrile ketone, polyether nitrile s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com