Method for preparing fuel cell catalyst
A fuel cell and catalyst technology, applied in the field of electrochemistry, can solve problems such as unfavorable catalyst particle size, Pt nanoparticle particle size growth, and control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention discloses a preparation method of a fuel cell catalyst, comprising the following steps:
[0033] H 2 PtCl 6 , the carrier and glucose are added to deionized water, the pH value is adjusted to 8-13, and a suspension is obtained after the reaction;
[0034] Add NaBH to the suspension 4 Solution, after the reaction, a fuel cell catalyst is obtained.
[0035] The present invention uses NaBH 4 As reducing agent, using NaBH 4 H 2 PtCl 6 Reduction gives Pt atoms. At the same time, glucose is used as a protective agent to control the interaction between the generated Pt atoms and the hydroxyl groups of glucose by adjusting the pH value to control the deposition of new Pt atoms, thereby controlling the particle size of the obtained fuel cell catalyst.
[0036] In the above-mentioned preparation steps for obtaining the suspension, H 2 PtCl 6 As a Pt source; the carrier is preferably activated carbon, carbon nanotubes, graphene or titanium dioxide; H 2 PtC...
Embodiment 1
[0052] To prepare Pt / C with a Pt content of 20wt%, the carrier Vulcan XC-72R was used.
[0053] Add 1.2g β-D-glucose (1.2g β-D-glucose) into 20mL deionized water to obtain a glucose solution; add 130mg NaBH 4 Dissolve in 50mL deionized water to get NaBH 4 solution;
[0054] 126mg of Vulcan XC-72R carrier was ultrasonically dispersed in 80mL of deionized water for 2 hours, and the glucose solution was added to the above ink under magnetic stirring; then, 30mL of precursor solution-chloroplatinic acid aqueous solution was added, containing 32mg of Pt element;
[0055] After stirring at room temperature for 4 hours, the NaBH was added dropwise 4 Solution, continue to stir for 8 hours, then use deionized water with a resistivity of 18.2MΩ cm to wash, and filter and wash until there is no Cl - exists, i.e. adding AgNO to the filtrate 3 There was no precipitation in the solution, and finally it was vacuum-dried at 80° C. to obtain a fuel cell catalyst, marked as Pt / C-a.
[0056...
Embodiment 2
[0061] Using the same preparation method as in Example 1, adjust the pH to 10 before adding the reducing agent.
[0062] The testing method and experimental conditions of this example are the same as those of Example 1, and the fuel cell catalyst prepared in Example 2 is marked as Pt / C-b.
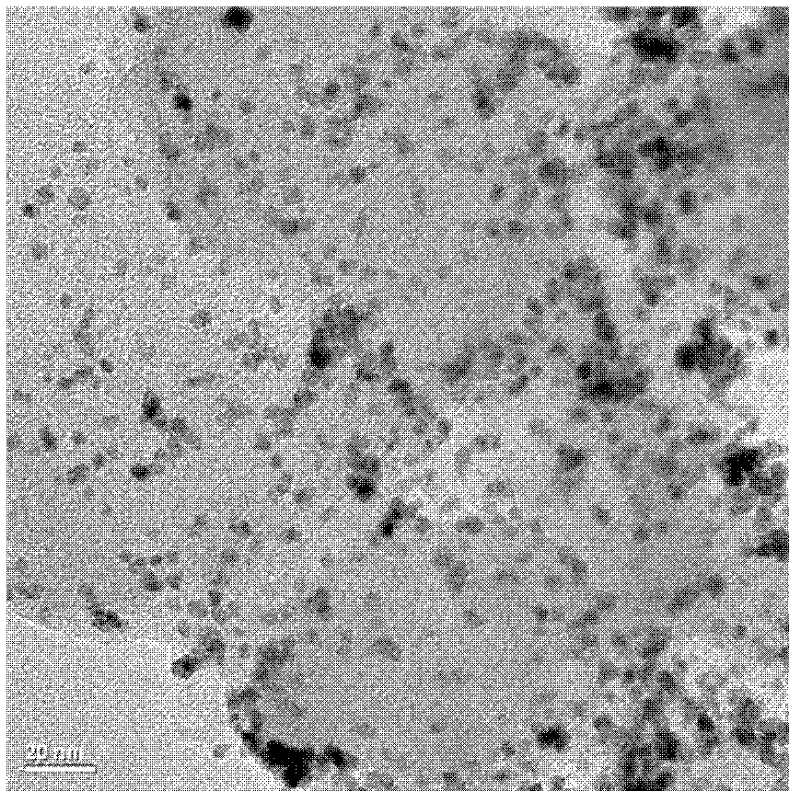
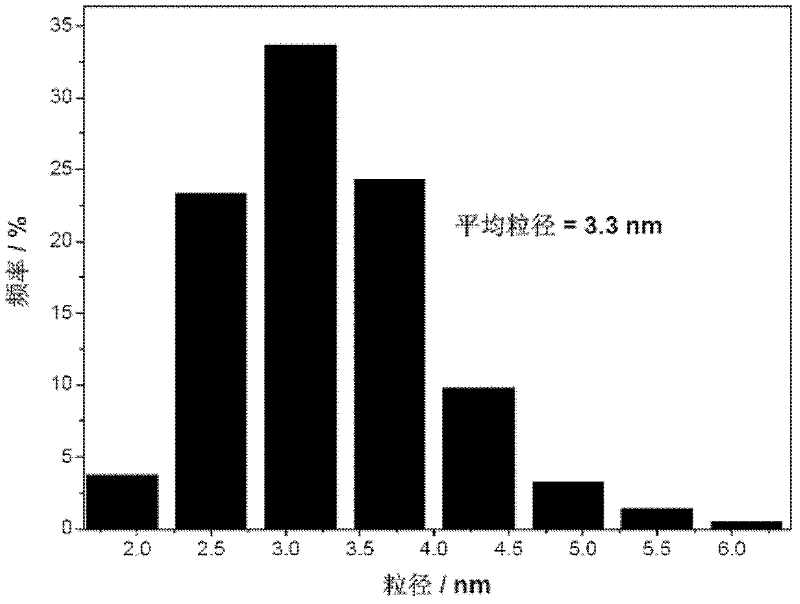
[0063] Figure 5 and Image 6 The transmission electron microscope pictures and particle size distribution diagrams of the fuel cell catalyst prepared for this example show that the average particle size is 2.7nm, and the distribution is narrow and the dispersion is good.
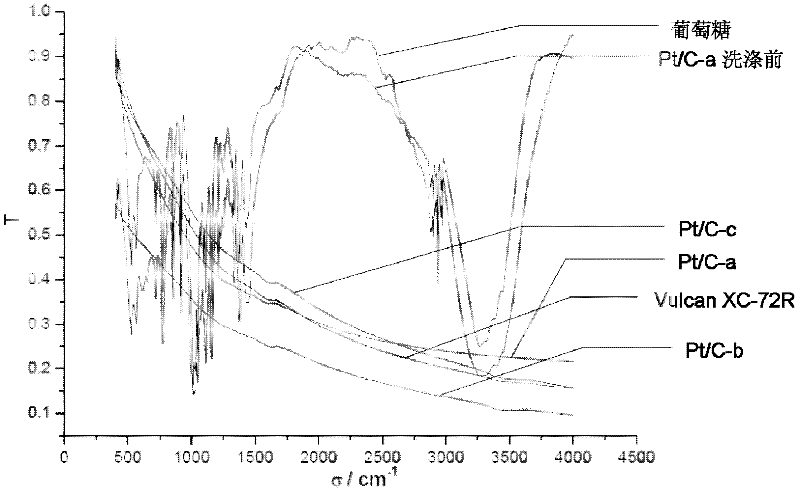
[0064] The fuel cell catalyst powder prepared in this example is ground and mixed with KBr and pressed into tablets for infrared transmission spectrum testing. The results are shown in image 3 .
[0065] The mass specific activity curve of the fuel cell catalysis prepared in this example is as follows Figure 4 Shown, the mass specific activity is 416.5mA / mg Pt .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistivity | aaaaa | aaaaa |
| Resistivity | aaaaa | aaaaa |
| Resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com