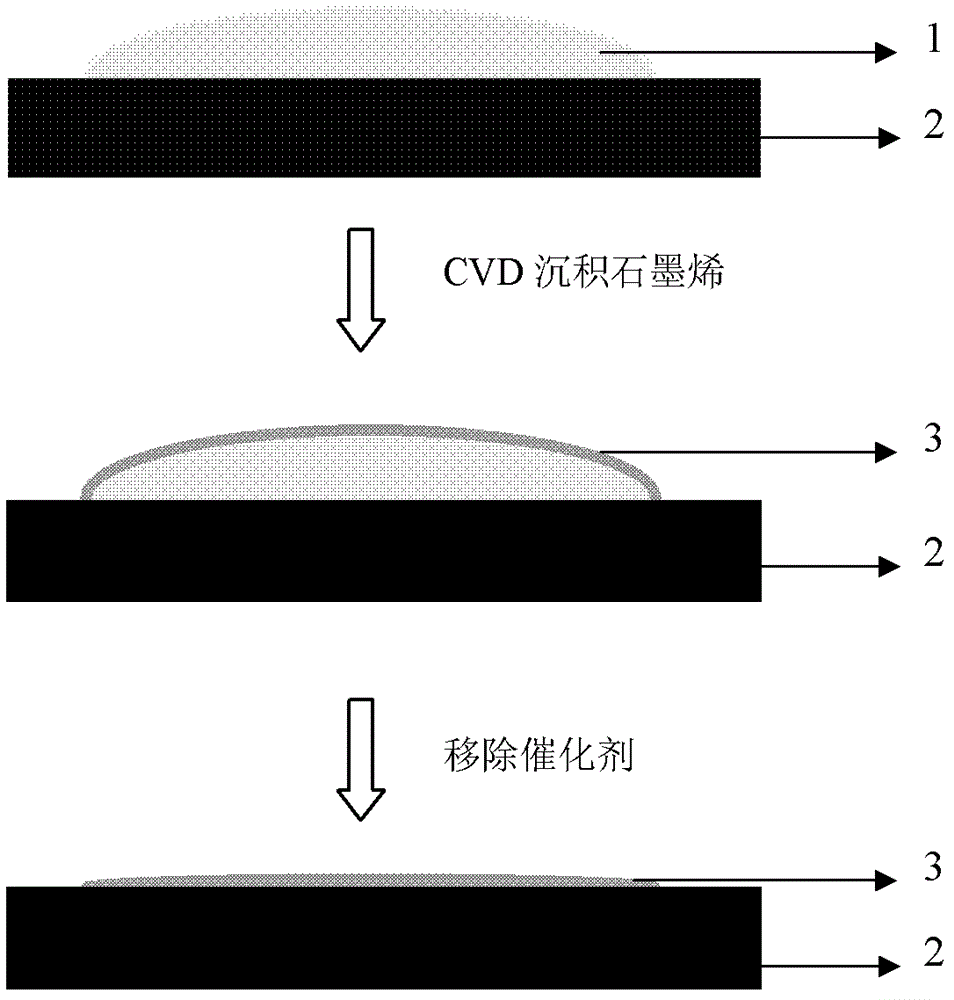
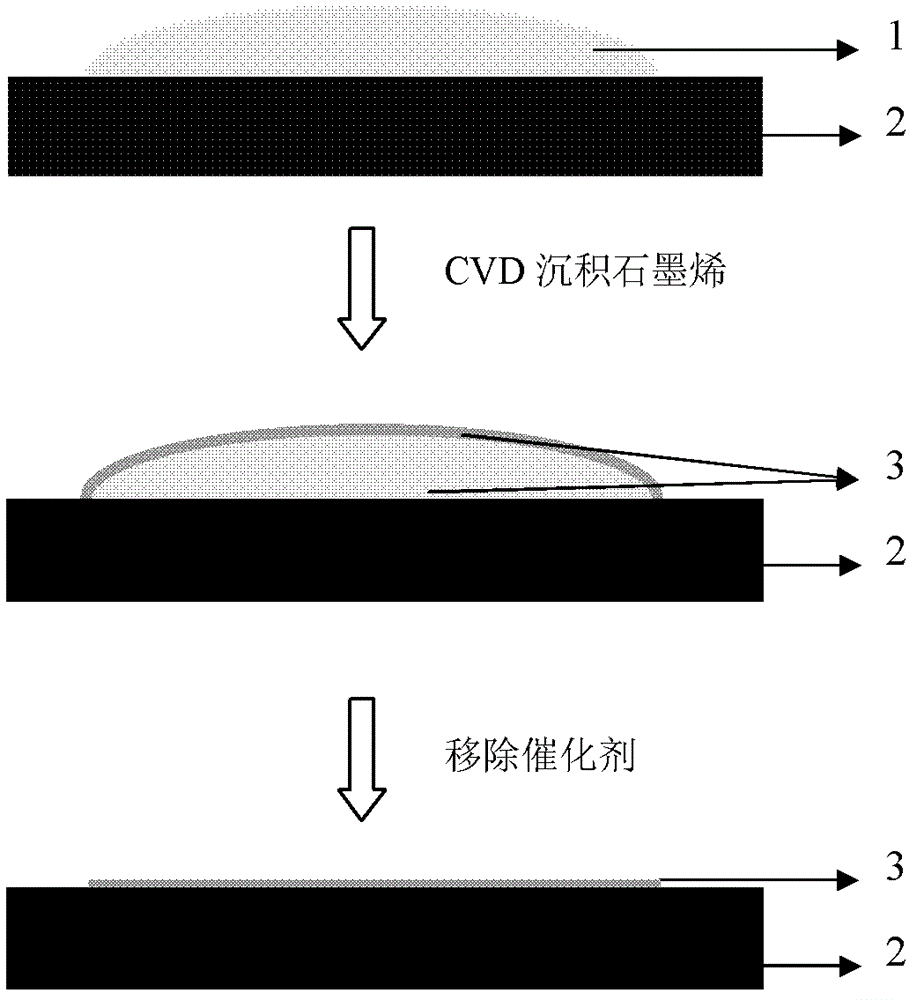
Method for preparing graphene by adopting liquid catalyst aided chemical vapor deposition
A chemical vapor deposition and graphene technology, applied in graphene, gaseous chemical plating, nanotechnology for materials and surface science, etc., can solve the problems of cumbersome transfer process, restricting the application of graphene, and graphene contamination , to improve the production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Using liquid gallium as a catalyst and solid carbon source to prepare graphene at low temperature
[0050] (1) Weigh 0.5 g of simple gallium with a purity of 99.999%, and place it on a gallium nitride substrate. Weigh 15 mg of solid carbon source naphthalene, put it in a quartz test tube with one end closed, and place it in the low-temperature heating zone of a tube furnace.
[0051] (2) Increase the temperature of the substrate, and the argon gas flow rate is 200 sccm as a protection. Simultaneously heat the carbon source to 150-200°C. When the substrate temperature reaches 500-700° C., the flow rate of hydrogen gas is 2 sccm, the flow rate of argon gas is 200 sccm, and the reaction time is 60 min.
[0052] Stop heating the liquid source and the tube furnace, and take out the sample after the chamber cools down to room temperature. The purity of the carrier gas used in the chemical gas phase reaction is higher than 99.999%.
[0053] (3) Freeze the sample...
Embodiment 2
[0055] Example 2: Using liquid gallium as a catalyst and gaseous carbon source to prepare graphene at high temperature
[0056] (1) Weigh 0.5 g of simple gallium with a purity of 99.999% and place it on an alumina substrate.
[0057] (2) The temperature of the substrate is raised with the argon flow rate of 200 sccm as a protection. When the furnace temperature rises to 1000° C., the methane flow rate is 2 sccm, the argon flow rate is 200 sccm, and the reaction time is 3 minutes. Stop heating the tube furnace, and take out the sample after cooling down to room temperature at a cooling rate greater than 30°C per second.
[0058] (3) The process of exfoliating graphene is similar to Example 1.
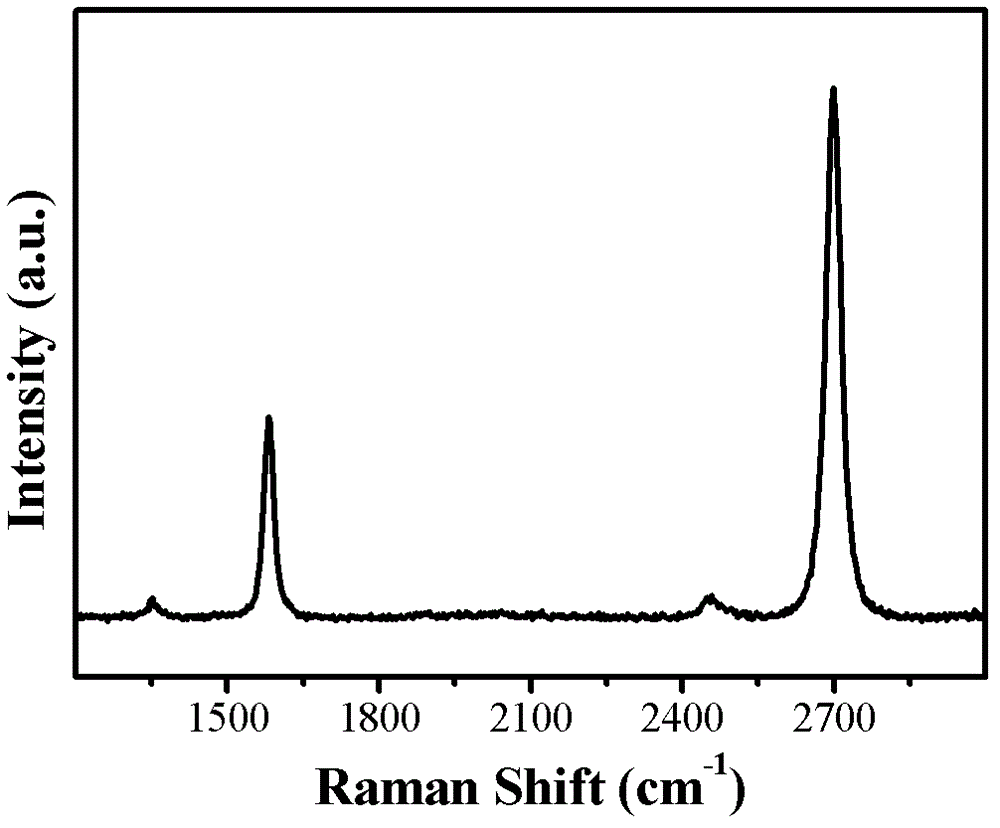
[0059] Example result: as Figure 4 It is shown that high-quality graphene is obtained in a short time at a higher temperature, and the 2D peak is located at 2700cm -1 Nearby, and twice the intensity of the G peak, indicating that the graphene is a single layer, and no defect peaks a...
Embodiment 3
[0060] Example 3: Using liquid gallium as a catalyst and gaseous carbon source to prepare graphene at high temperature
[0061] (1) Weigh 0.5 g of gallium elemental substance with a purity of 99.999%, and place it on a quartz substrate.
[0062] (2) The temperature of the substrate is raised with the argon flow rate of 200 sccm as a protection. When the furnace temperature rises to 1000° C., the methane flow rate is 5 sccm, the argon flow rate is 200 sccm, and the reaction time is 30 minutes. Stop heating the tube furnace and remove the sample after the chamber has cooled to room temperature.
[0063] (3) The process of exfoliating graphene is similar to Example 1.
[0064] Example result: as Figure 5 It is shown that thicker graphene is obtained at higher temperature for a longer time, and the 2D peak is located at 2700cm -1 Nearby, it is about 0.8 times the intensity of the G peak, indicating that graphene is multi-layered, and a smaller defect peak appears. Through hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com