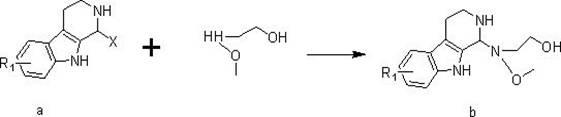
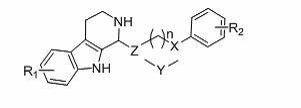
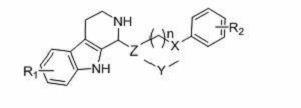
Pyridoindole derivative and antibacterial application thereof
A technology of indole derivatives, which is applied in the field of pyridoindole derivatives and their antibacterial applications, achieving the effects of mild reaction conditions, easy availability of raw materials, and abundant raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Active compounds were screened by computer-aided virtual screening method. Using the three-dimensional structure of the complex of the first extreme thermophilic bacterial RNA polymerase and the inhibitor acting on the active site of the "switch area" published on Cell in 2008, the active site of the "switch area" was selected. Use Surflex to conduct a three-dimensional database search to conduct a rough screening of 50,000 compounds and derivatives in the natural product library, and then select the compounds with a score of 7 or more in the docking results, and then use Gold and Autodock to perform detailed screening respectively, and obtain 20 compounds Molecules with higher scoring and binding energy were combined with drug-like analysis and visual observation to remove obviously unreasonable ones, and finally 6 compounds were selected to enter the next step of the experiment. The scoring values of these six compounds are shown in Table 1.
[0051] Table 1: Scori...
Embodiment 2
[0054] N-((4-fluorobenzyloxy)methyl)-O-methyl-N-(6-(trifluoromethyl)-2,3,4,9-tetrahydropyridoindole)hydroxylamine, etc. In vitro inhibition experiment of 6 compounds on bacterial RNA polymerase. For N-((4-fluorobenzyloxy)methyl)-O-methyl-N-(6-(trifluoromethyl)-2,3,4,9-tetrahydropyridoindole)hydroxylamine Molecules, by detecting the reduction of substrate ATP——Kool? NC-45? RNAP Activity & Inhibitor Screening Kit (Epicentre Company): 2 μg of E. Pyridoindole derivatives were reacted at 25°C for 20 minutes, and then 1002 μM ATP (total reaction volume 50 μL) was added. After acting at 25°C for 20 minutes, the reaction mixture was added to a 96-well microtiter plate, 50 μL per well, and Kool? NC- 50 μL of 45? Reagent per well was left standing at room temperature for 10 minutes, and the chemiluminescence value was read to indicate the amount of ATP remaining in the reaction. The experiment without compound was set as the control group. Finally calculate N-((4-fluorobenzyloxy)methy...
Embodiment 3
[0061] N-(4-(5-(6-(Benzyloxy)-2,3,4,9-tetrahydropyridindole)-2-methoxybenzyloxy)phenyl)acetamide etc.6 In vitro inhibition experiments of compounds on the growth of standard bacteria
[0062] Use the standard tube dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI):
[0063] 1. Inoculate the bacteria in fresh MH liquid medium and culture overnight at 37°C;
[0064] 2. Correct the bacterial solution to the 0.5 McFarland turbidity standard with fresh MH liquid medium, then dilute it with MH liquid medium at 1:200, add 1 mL to each test tube, and add 1 mL of N-( (4-fluorobenzyloxy)methyl)-O-methyl-N-(6-(trifluoromethyl)-2,3,4,9-tetrahydropyridoindole)hydroxylamine and other 6 compounds (The final concentration of solvent DMSO is maintained at 1%), cultured at 37°C for 18 hours, with 1% DMSO + bacteria as the control, and sterile medium as the blank control;
[0065] 3. Take out the tube with the lowest concentration of bacteria that does not g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com