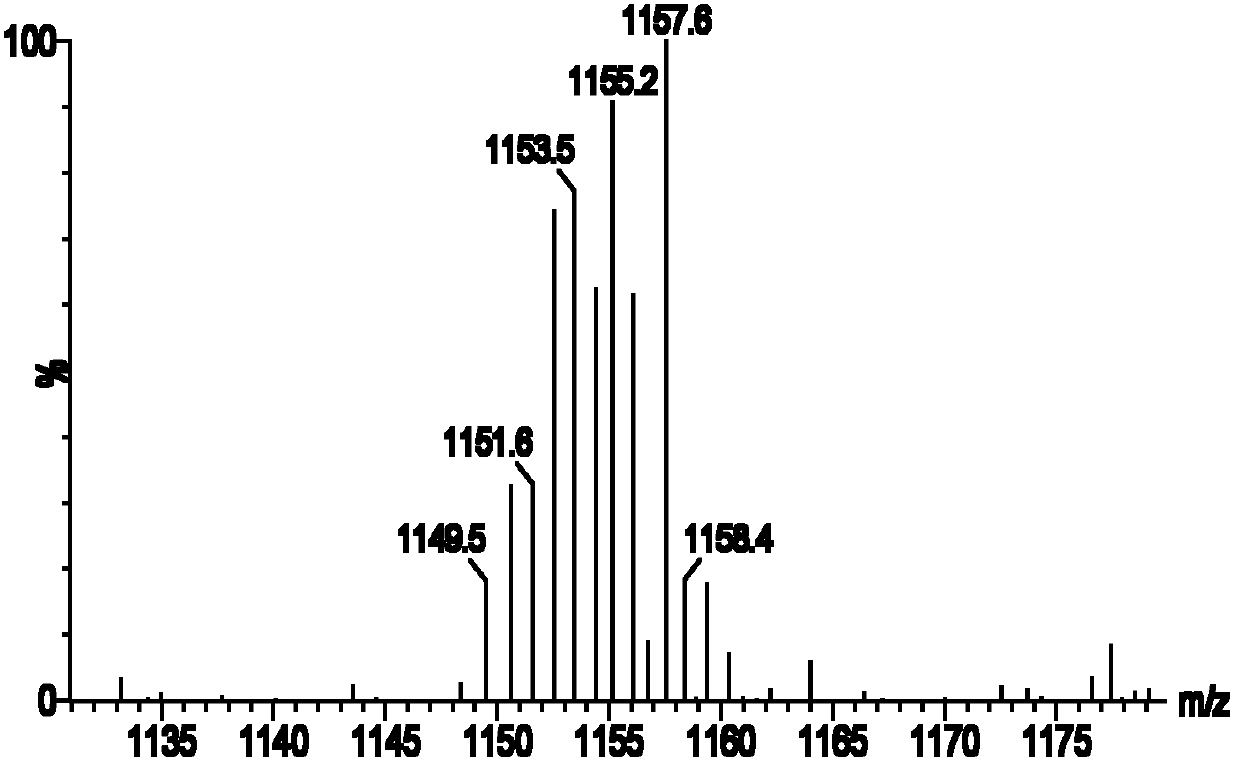
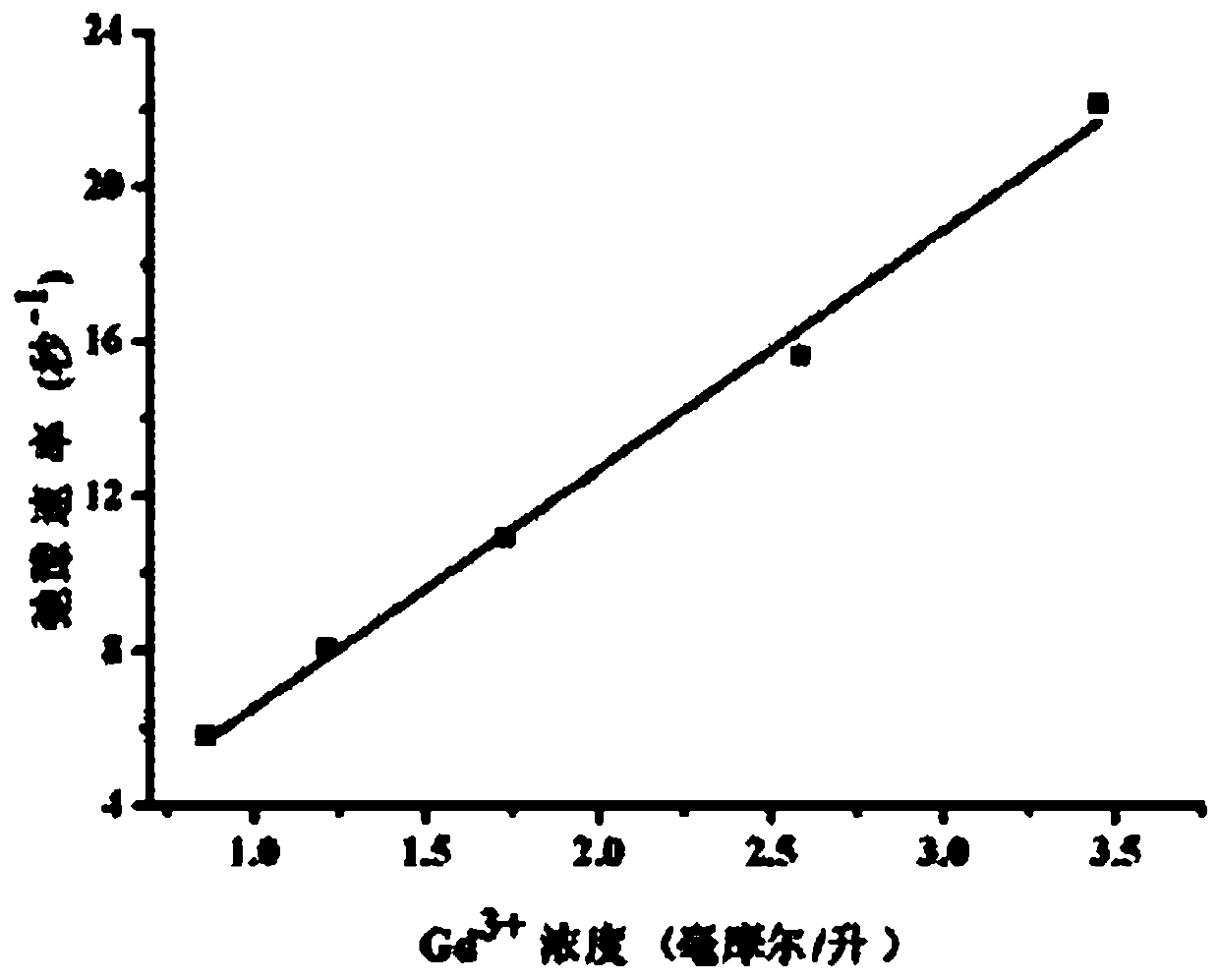
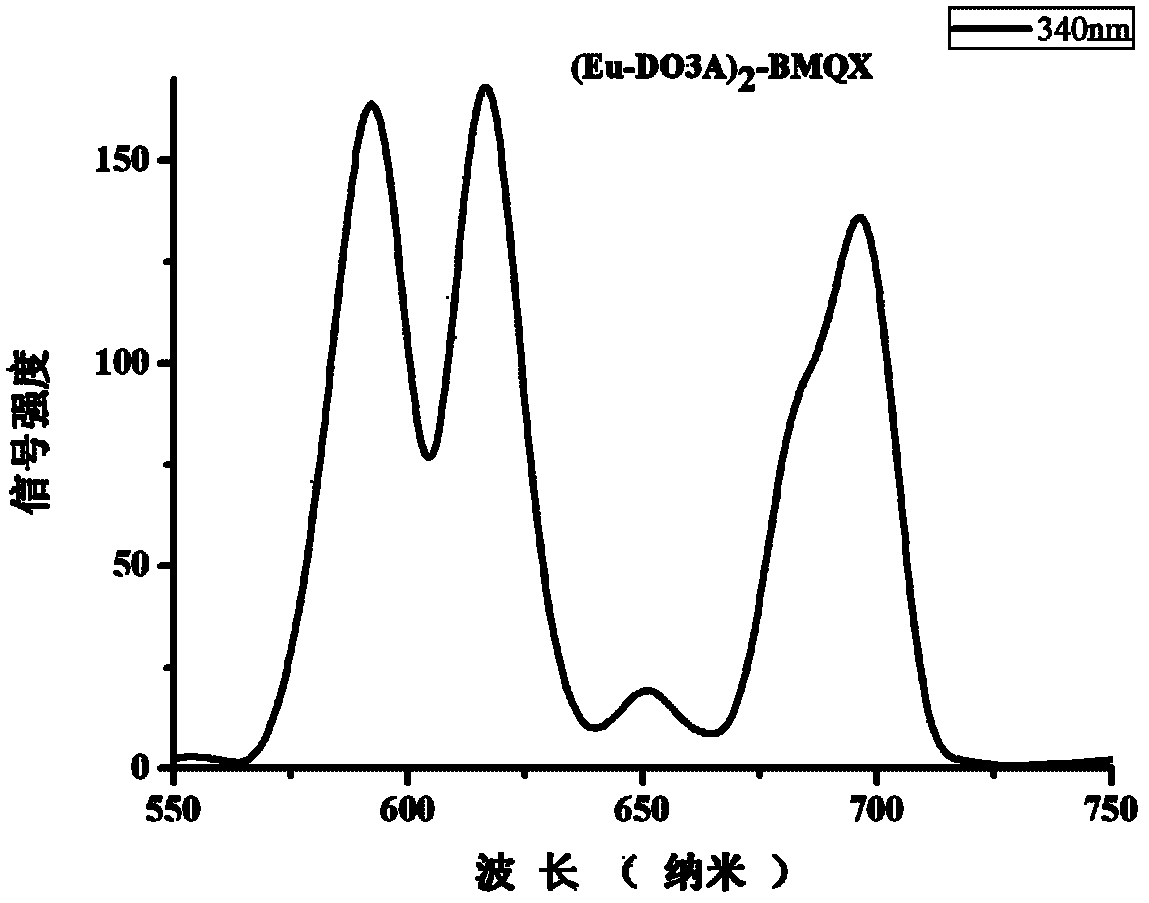
2-nuclear magnetic resonance imaging contrast agent taking 2, 3-biquinoxaline as connecting body and preparation method thereof
A nuclear magnetic resonance imaging and quinoxaline technology, which is applied to preparations, chemical instruments and methods for in vivo tests, compounds containing elements of Group 3/13 of the periodic table, etc., can solve the problem of poor thermodynamic stability and low relaxation efficiency. and other problems, to achieve the effect of favorable storage, good water solubility, and improved thermodynamic and kinetic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 (Gd-DO3A) 2 - Synthesis of BMQX
[0048] Step 1: Synthesis of 2,3-bis(bromomethyl)quinoxaline
[0049] Under nitrogen protection, add 0.681 g of o-phenylenediamine to a 50 ml round bottom flask, add 4 ml of anhydrous tetrahydrofuran to dissolve, stir at 5°C, add 1.46 g of 1,4-dibromo-2,3- 8 milliliters of tetrahydrofuran solution of diacetyl, obtain mixed solution, allow mixed solution rise to room temperature gradually, stop reaction after continuing to react for 3 hours, remove solvent tetrahydrofuran under reduced pressure distillation, obtain reaction product, product carries out chromatographic separation with silica gel column, The eluent was a mixture of ethyl acetate and petroleum ether at a volume ratio of 1:12, and the eluent was evaporated to obtain 1.67 g of 2,3-bis(bromomethyl)quinoxaline as a white solid;
[0050] Step two: ( t Bu-DO3A) 2 - Synthesis of BMQX
[0051] Under nitrogen protection, 1.22 g t Bu-DO3A HBr, 0.682 g NaHCO 3 Add it t...
Embodiment 2
[0058] Embodiment 2, (Gd-DO3A) 2 - Synthesis of BMQX
[0059] Step 1: Synthesis of 2,3-bis(bromomethyl)quinoxaline
[0060] Under nitrogen protection, add 0.681 g of o-phenylenediamine to a 50 ml round bottom flask, add 4 ml of anhydrous tetrahydrofuran to dissolve, stir at 10°C, add 1.46 g of 1,4-dibromo-2,3- 8 milliliters of tetrahydrofuran solution of diacetyl, to obtain mixed solution, allow mixed solution to rise to room temperature gradually, stop reaction after continuing to react for 2 hours, remove solvent tetrahydrofuran under reduced pressure distillation, obtain reaction product, product carries out chromatographic separation with silica gel column, The eluent was a mixture of ethyl acetate and petroleum ether at a volume ratio of 1:12, and the eluent was evaporated to obtain 1.67 g of 2,3-bis(bromomethyl)quinoxaline as a white solid;
[0061] Step two: ( t Bu-DO3A) 2 - Synthesis of BMQX
[0062] Under nitrogen protection, 1.22 g t Bu-DO3A HBr, 0.682 g NaHCO ...
Embodiment 3
[0075] Example 3 (Gd-DO3A) 2 - Synthesis of BMQX
[0076] Step 1: Synthesis of 2,3-bis(bromomethyl)quinoxaline
[0077] Under nitrogen protection, add 2.724 grams of o-phenylenediamine to a 100 ml round bottom flask, add 16 ml of anhydrous tetrahydrofuran to dissolve, stir at 0°C, add 5.84 g of 1,4-dibromo-2,3- 32 milliliters of tetrahydrofuran solution of diacetyl, to obtain mixed solution, remove the ice-water bath, allow mixed solution to rise to room temperature gradually, stop reaction after continuing to react for 4 hours, remove solvent tetrahydrofuran under reduced pressure distillation, obtain reaction product, product is carried out with silica gel column It was separated by chromatography, and the eluent was a mixture of ethyl acetate and petroleum ether at a volume ratio of 1:12. The eluent was evaporated to obtain 6.8 g of white solid 2,3-bis(bromomethyl)quinoxaline;
[0078] Step two: ( t Bu-DO3A) 2 - Synthesis of BMQX
[0079] Under nitrogen protection, 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com