Flame-retardant lithium ion battery electrolyte and method for preparing same
A lithium-ion battery, flame-retardant technology, applied in the direction of secondary batteries, circuits, electrical components, etc., can solve the problems of poor compatibility with the negative electrode, low cost of synthesis, good wettability, good The effect of flame retardancy and even non-combustibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic method of N, N-diallyl-diethoxyphosphoramide (DEDAPA):
[0048] In a 250mL three-necked flask, 8.63g (50mmol) of diethoxyphosphoryl chloride and 8.5mL (60mmol) of triethylamine were dissolved in 100mL of anhydrous acetonitrile, and 5.8g (60mmol) of diallyl The amine was reacted at room temperature for 5 h, extracted, and the organic phase was collected, dried, and distilled under reduced pressure to obtain 11.2 g of the product, with a yield of 96%. Using ESI-MS, 1 HNMR, 13 C NMR and 31 The product was characterized by P NMR, and the result confirmed that it was the target product. Data are as follows:
[0049] 1 H NMR (CDCl 3 , 400MHz): δ5.81-5.68(m, 2H), 5.21-5.12(m, 4H), 4.12-3.96(m, 4H), 2.55(d, J=9.6Hz, 4H), 1.06(t, J = 7Hz, 6H); 13 C NMR (CDCl 3 , 100MHz): δ134.5, 117.7, 62.1 (d, J C-P =6.2Hz), 47.6(d, J C-P =4.2Hz), 16.1(d, J C-P =7.3Hz); 31 P NMR (CDCl 3 , 162MHz): δ13.49.ESI-MS: m / z=234[M+H] + ;256[M+Na] + .
Embodiment 2
[0051] N-methyl-N-allyldiethoxyphosphoramide (DEAMPA) synthetic method 1:
[0052] In a 250mL three-necked flask, 8.63g (50mmol) of diethoxyphosphoryl chloride and 8.5mL (60mmol) of triethylamine were dissolved in 100mL of anhydrous acetonitrile, and 4.3g (60mmol) of methallyl was added dropwise under ice-cooling conditions. base amine, reacted at room temperature for 5 h, extracted, collected the organic phase, dried, and distilled under reduced pressure to obtain 9.8 g of the product, with a yield of 95%.
[0053] N-methyl-N-allyldiethoxyphosphoramide (DEAMPA) synthetic method two:
[0054] In a 250 mL three-necked flask, dissolve 8.63 g (50 mmol) of diethyl hydrogen phosphite, 8.5 mL (60 mmol) of triethylamine and 4.3 g (60 mmol) of methallylamine in 80 mL of acetonitrile, and slowly Slowly add 23g of carbon tetrachloride dropwise, react at room temperature for 5h, extract, collect the organic phase, dry, and distill under reduced pressure to obtain 9.3g of the product, wi...
Embodiment 3
[0059] The alkenylphosphoramide contained in this example is a phosphorus-containing organic compound with the following structural formula:
[0060]
[0061] That is, the R in the general formula 1 , R 2 Both are ethyl, R 3 , R 4 Allyl, the above compound named N, N-diallyl-diethoxyphosphoramide, referred to as DEDAPA.
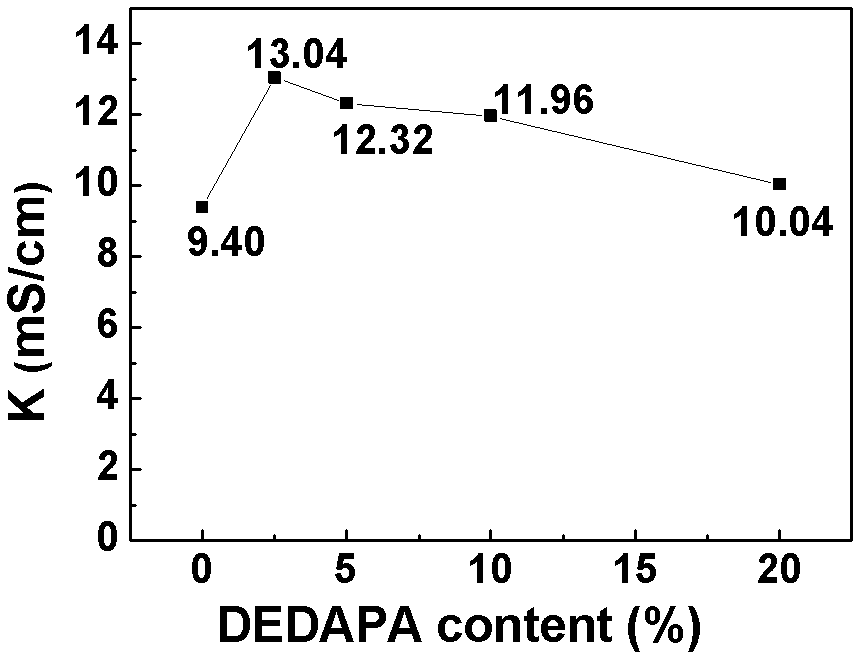
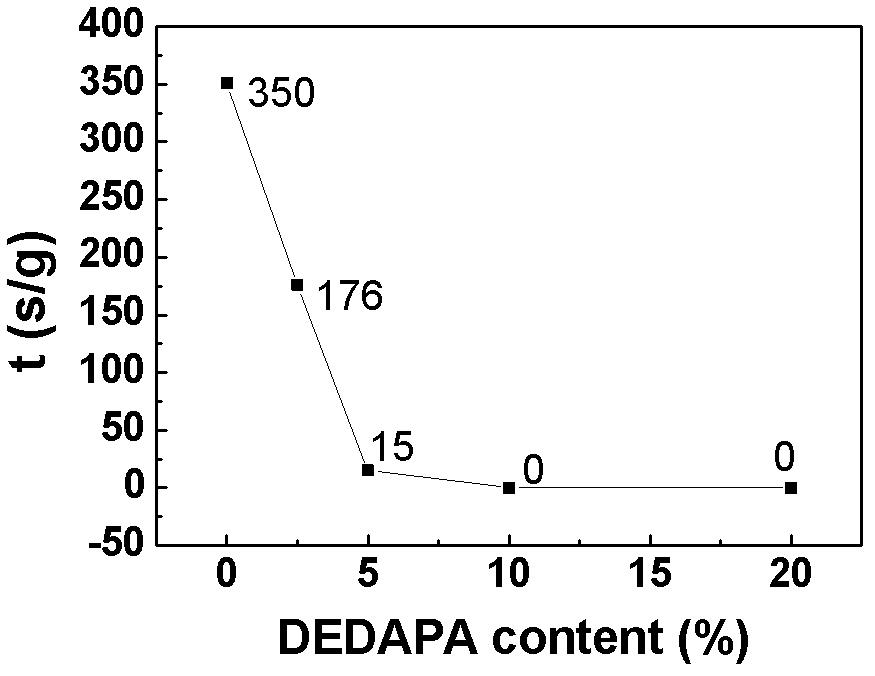
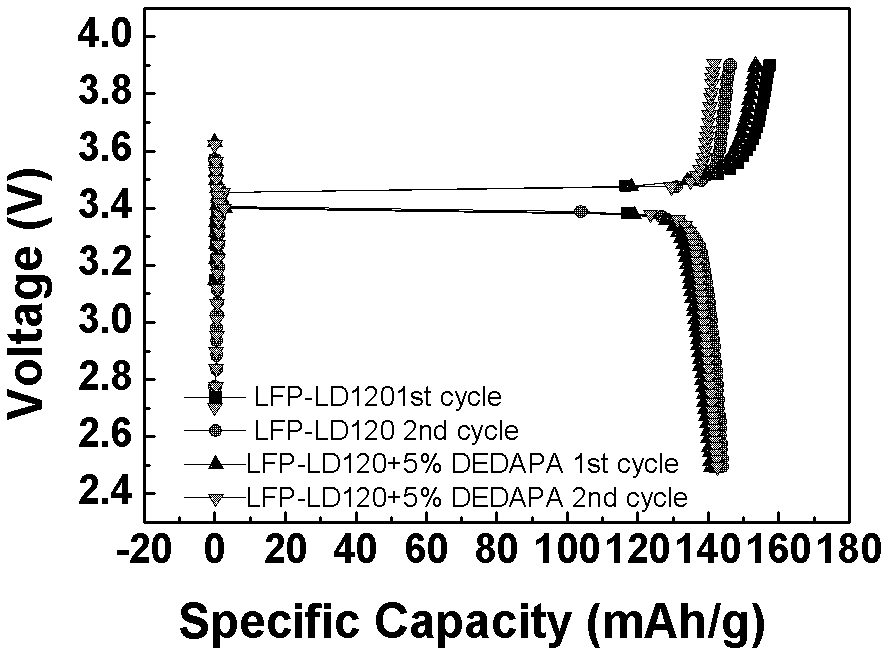
[0062] lithium salt LiPF 6 Soluble in solvent (EC:DMC=1:1) and additive DEDAPA, in which LiPF 6 Accounting for 10%, the content of DEDAPA is 2.5%, 5%, 10%, 20% respectively, the rest is solvent, and the electrolytes are respectively named 2.5% DEDAPA, 5% DEDAPA, 10% DEDAPA, 20% DEDAPA; and DEDAPA accounts for 10% , LiPF 6 5%, 10%, 20%, and the rest are solvent electrolytes, named DEDAPA-5% LiPF respectively 6 , DEDAPA-10%LiPF 6 , DEDAPA-20%LiPF 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com