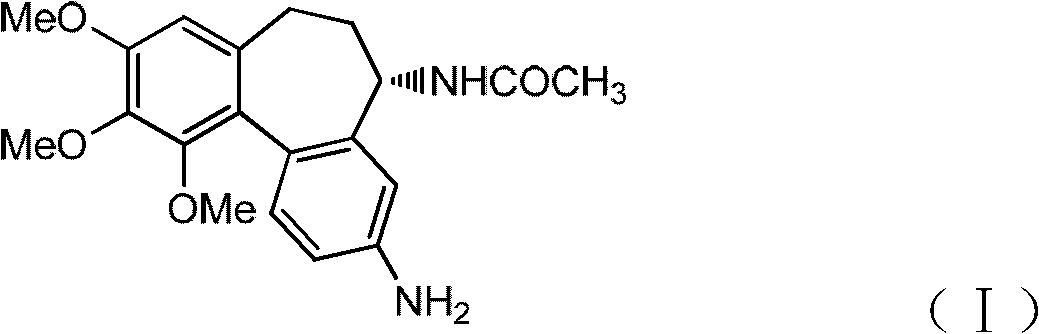
Method for preparing intermediate of colchicine derivatives
A technology for colchicine and derivatives, which is applied in the field of preparation of colchicine derivative intermediates, can solve the problems of restricting new drug research and development, failing to obtain products, reducing product yields, etc., and achieve large-scale industrial production and reaction conditions Gentle, Process-Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
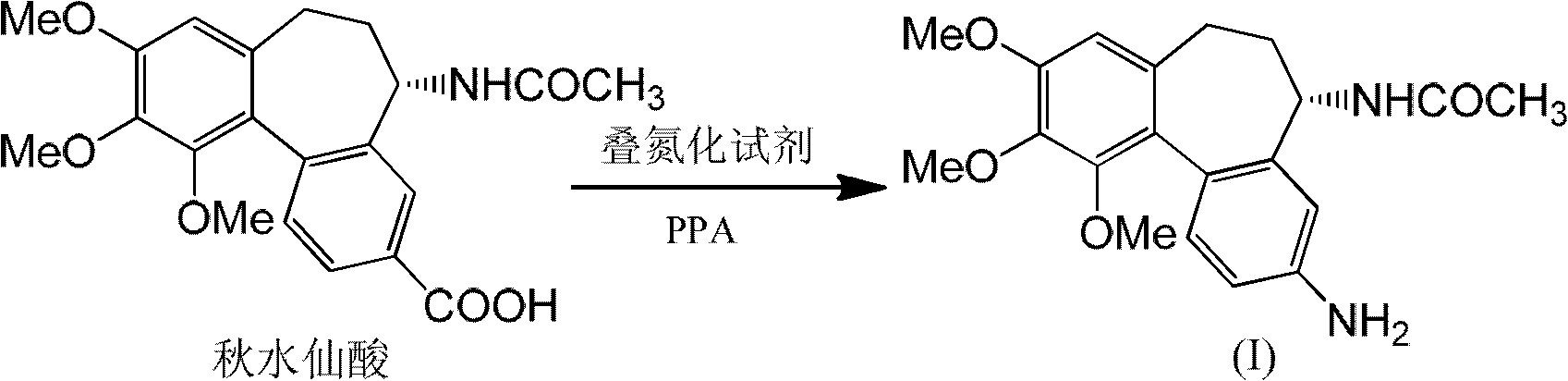
[0025] 7.0g colchicic acid was added to 70g PPA (85%P 2 o 5 ), that is, polyphosphoric acid (containing 85% phosphorus pentoxide), heated to 100°C, and 8.5g of sodium azide was added in batches while vigorously stirring. The gas generated during the reaction can be absorbed with cold alkaline solution.
[0026] After reacting until there is basically no gas evolution, follow the reaction with TLC (chloroform / methanol volume ratio 10:1) until the colchicic acid is consumed. Cool in a water bath to 60°C, add 100ml of water, stir until the PPA solution is completely dissolved, pour it into a beaker containing 500 grams of crushed ice, and adjust the pH to neutral or weakly alkaline with an aqueous solution with a molar concentration of 5M NaOH.
[0027] Suction filtration was performed after adjusting the pH value, and the obtained solid was washed with water until the water phase was neutral, and 4.2 g of solid was obtained after drying;
[0028] The filtrate obtained by suct...
Embodiment 2
[0031] Adopt the experimental condition identical with embodiment 1, only PPA (85%P 2 o 5 ) to PPA (80%P 2 o 5 ). The resulting solid crude product was recrystallized with methanol-water at a volume ratio of 2:1, and dried to obtain (5S)-N-(3-amino-9,10,11-trimethoxy-6,7-dihydro-5H - Dibenzo[a,c]cyclohepten-5-yl)acetamide 4.2 g.
Embodiment 3
[0033] 7g P 2 o 5 Add 5g 90% H in 3 PO 4 , Heated and stirred at 120 ° C, now made of polyphosphoric acid. Add 4.5 g of colchicic acid, then dropwise add 60 mL of 0.5 mol / L azide in benzene solution, heat to reflux, follow the reaction with TLC (chloroform / methanol volume ratio 10:1), until the colchicic acid is consumed , the solvent benzene was removed by rotary evaporation, and 80ml of water was added to make the polyphosphoric acid solution fully dissolved, and the follow-up treatment was the same as in Example 1 to obtain (5S)-N-(3-amino-9,10,11-trimethoxy-6, 2.8 g of 7-dihydro-5H-dibenzo[a,c]cyclohepten-5-yl)acetamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com