Method for preparing tetanus human immune globulin by double virus inactivation
A technology of human immunoglobulin and immunoglobulin, applied in the direction of anti-bacterial immunoglobulin, immunoglobulin from serum, peptide preparation method, etc., can solve the problems affecting the ability to remove viruses, and achieve the effect of reducing diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
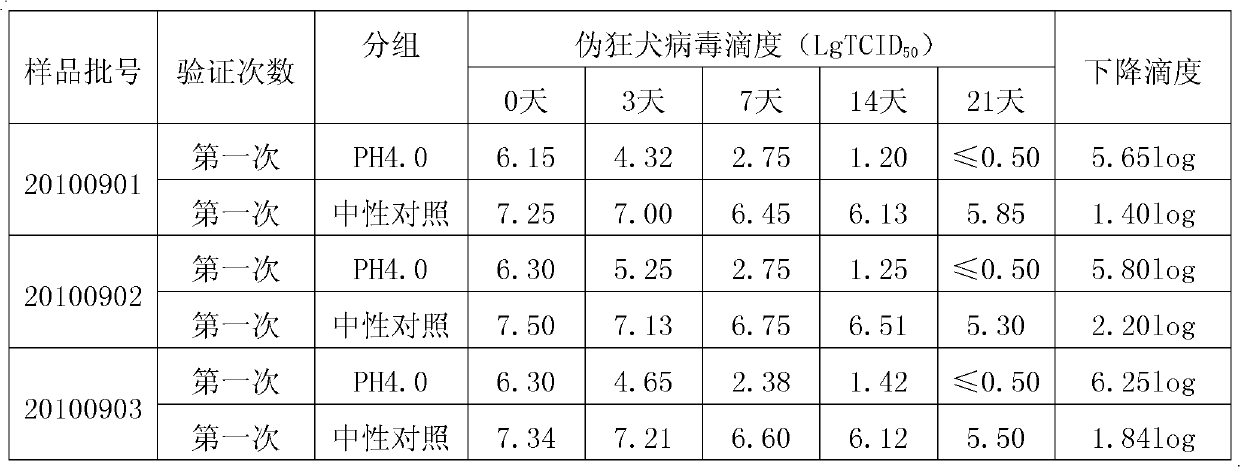
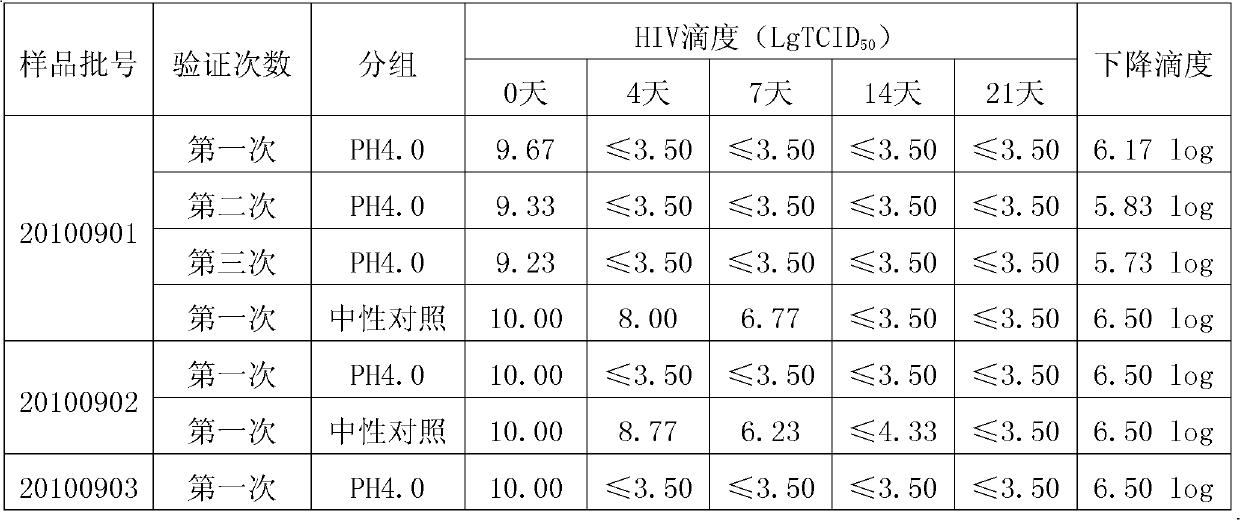
[0025] This strain is separated from healthy human plasma containing high titer tetanus antibody, separated and purified by low-temperature ethanol protein separation method, and used pH4.0, 23~25℃21 days incubation inactivated virus and DV50 nanomembrane in addition to virus membrane. Virus inactivation production process system. It is mainly used to prevent and treat tetanus, especially for those who have allergic reactions to tetanus antitoxin (TAT).
[0027] Take out qualified raw plasma for more than 100 people, and melt it after cleaning and disinfection. The melted plasma is combined in the reaction tank, and the plasma in the reaction tank is measured. The plasma in the reaction tank is evenly stirred and then sampled for testing of total protein content, albumin purity and tetanus antibody.
[0028] 2. Component I+II+III(F I+II+III )
[0029] 2.1. Component I (F I )
[0030] The plasma was diluted with 0.14mol / L NaCl solution, diluted and mixed. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com