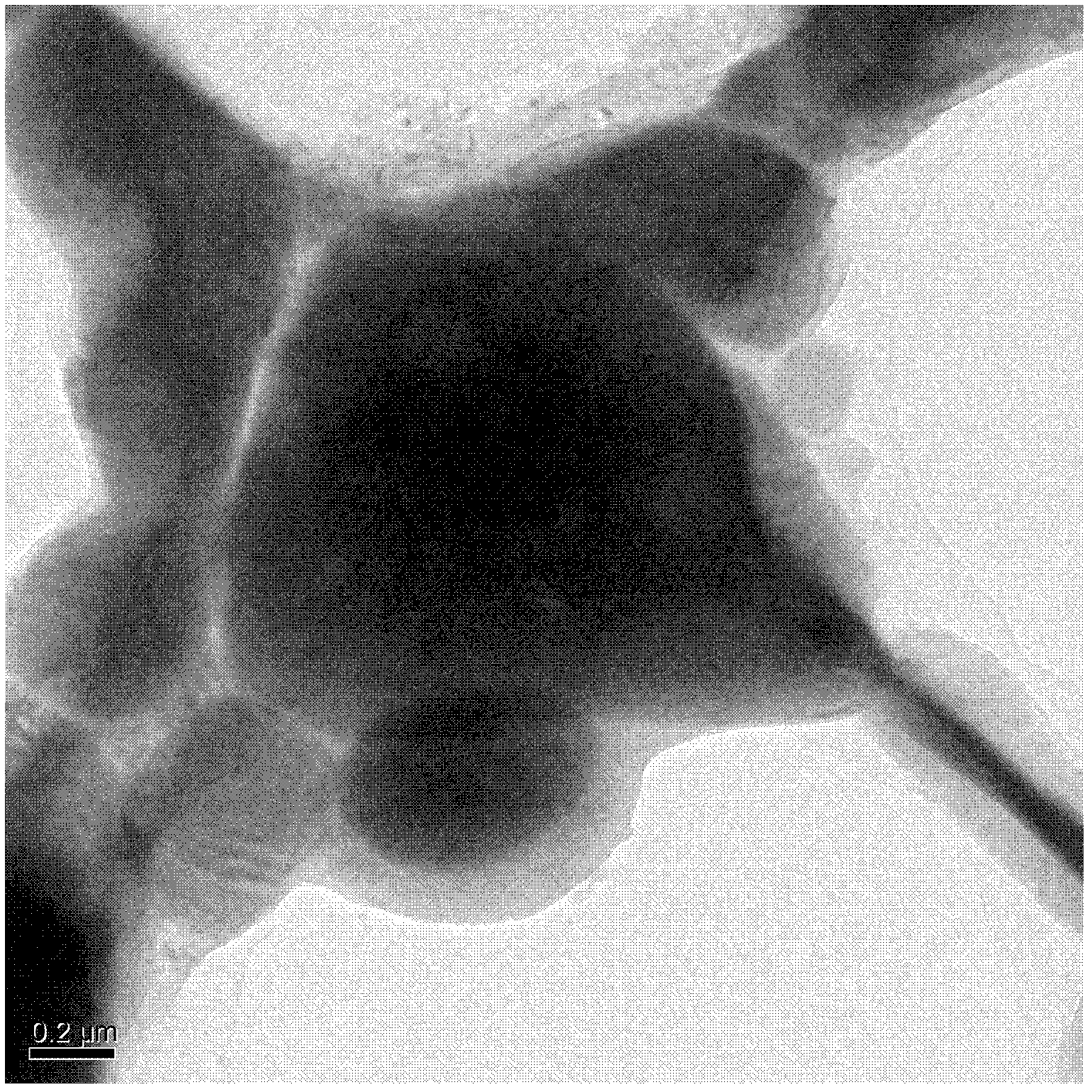
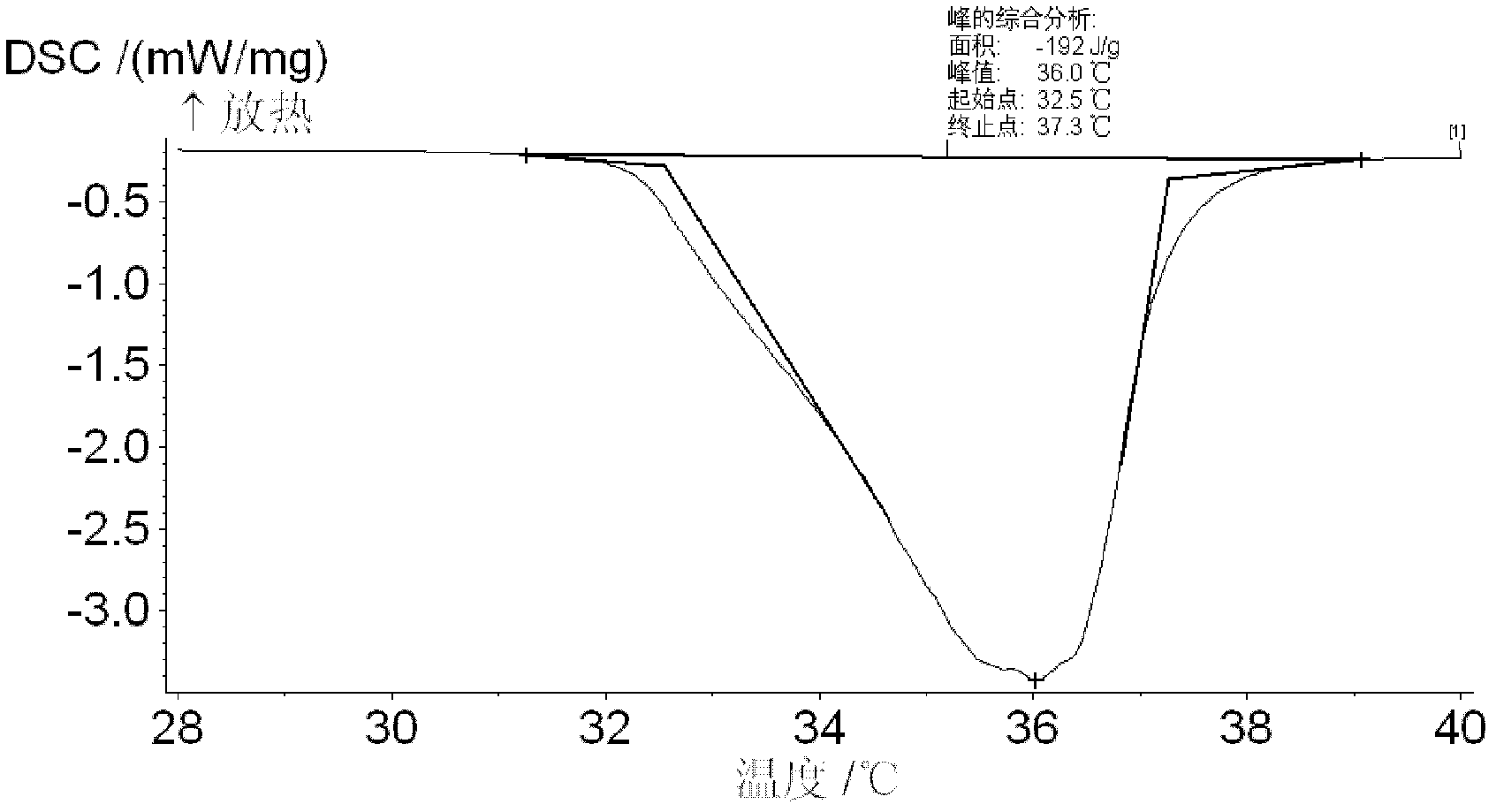
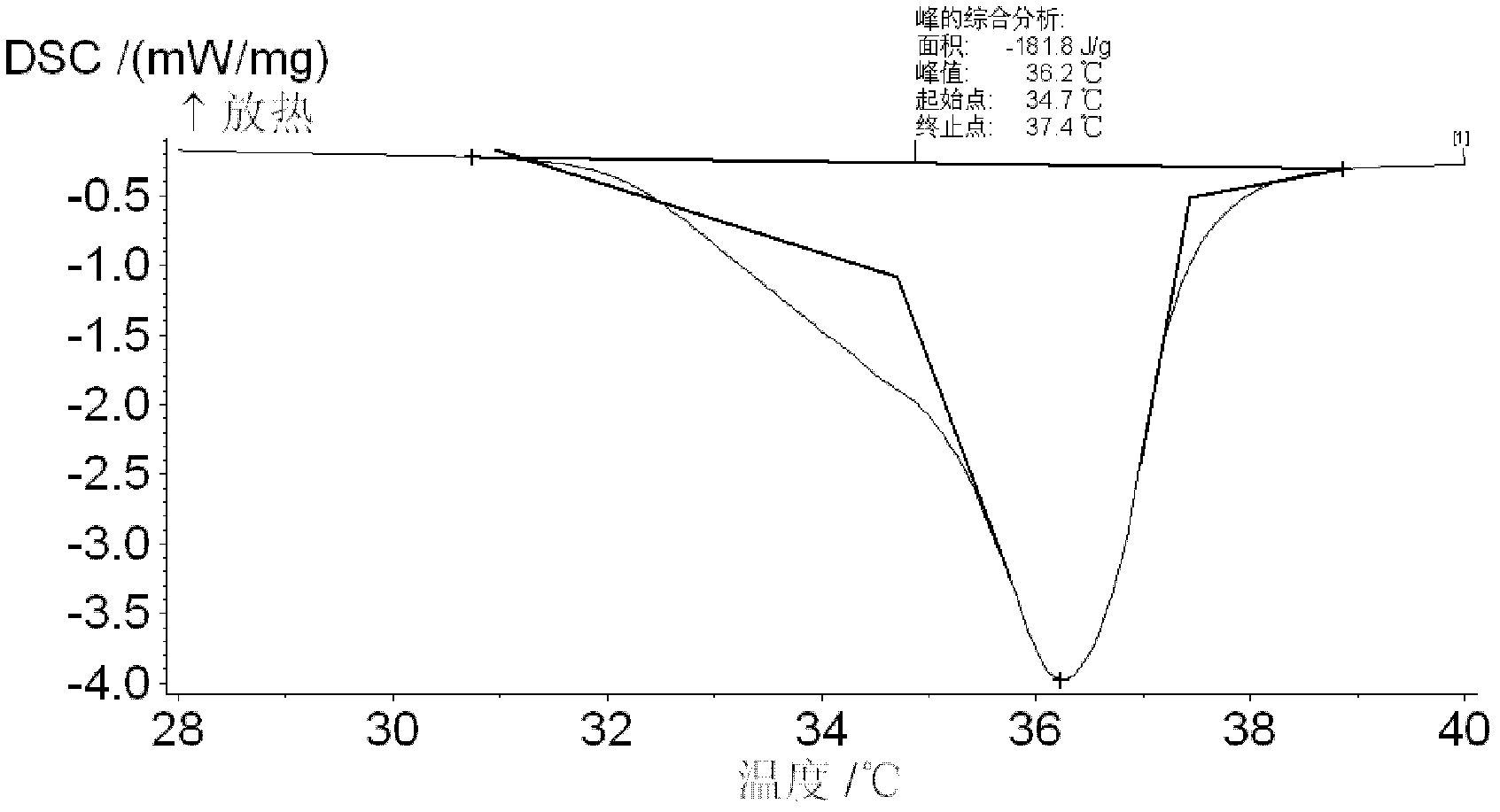
Phase change energy storage material of microscale polyvinyl chloride coated sodium sulfate decahydrate and preparation method of phase change energy storage material
A phase-change energy storage material, polyvinyl chloride technology, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of complicated preparation methods, serious phase separation, and long time consumption, and achieve low price and high preparation method The effect of simplicity and few operation links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Preparation: Weigh 0.15g of polyvinyl chloride into a clean grinding bottle, and add magnetron and 20ml tetrahydrofuran into the grinding bottle, stir the solution from milky to clear solution ①, set aside;
[0038] Measure 2ml of oleic acid into a clean beaker, add 10ml of absolute ethanol to the beaker, shake the beaker slightly until the two solutions are miscible, and obtain a mixed solution of oleic acid-ethanol for later use;
[0039] 2. Reaction process: weigh 4.0g Na 2 SO 4 In a clean small beaker, add a stirring magnet and 10ml deionized water into the beaker, stir in a constant temperature water bath at 33°C until completely dissolved; then the saturated Na 2 SO 4 The solution was placed in a water bath at 16°C, and at the same time, 1.5ml of the prepared oleic acid-ethanol mixed solution was added to Na 2 SO 4 Add dropwise to the solution and stir while dripping to obtain a large amount of white solid. Put the white solid and the solution into a centri...
Embodiment 2
[0041] 1. Preparation: Weigh 0.15g of polyvinyl chloride into a clean grinding bottle, and add magnetron and 20ml tetrahydrofuran into the grinding bottle, stir the solution from milky to clear solution ①, set aside;
[0042] Measure 2ml of oleic acid into a clean beaker, add 10ml of absolute ethanol to the beaker, shake the beaker slightly until the two solutions are miscible, and obtain a mixed solution of oleic acid-ethanol for later use;
[0043] 2. Reaction process: weigh 4.0gNa 2 SO 4 In a clean small beaker, add a stirring magnet and 10ml deionized water into the beaker, stir in a constant temperature water bath at 33°C until completely dissolved; then the saturated Na 2 SO 4 Put the solution in a water bath at 16°C, and at the same time take 1.75ml of prepared oleic acid-ethanol mixture to Na 2 SO 4 Add dropwise to the solution and stir while dripping to obtain a large amount of white solid. Put the white solid and the solution into a centrifuge tube and centrifuge...
Embodiment 3
[0046] 1. Preparation: Weigh 0.15g of polyvinyl chloride into a clean grinding bottle, and add magnetron and 20ml tetrahydrofuran into the grinding bottle, stir the solution from milky to clear solution ①, set aside;
[0047] Measure 2ml of oleic acid into a clean beaker, add 10ml of absolute ethanol to the beaker, shake the beaker slightly until the two solutions are miscible, and obtain a mixed solution of oleic acid-ethanol for later use;
[0048] 2. Reaction process: weigh 4.0gNa 2 SO 4 In a clean small beaker, add a stirring magnet and 10ml deionized water into the beaker, stir in a constant temperature water bath at 33°C until completely dissolved; then the saturated Na 2 SO 4 The solution was placed in a water bath at 20°C, and at the same time, 1.5ml of the prepared oleic acid-ethanol mixture was added to Na 2 SO 4 Add dropwise to the solution and stir while dripping to obtain a large amount of white solid. Put the white solid and the solution into a centrifuge tub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com