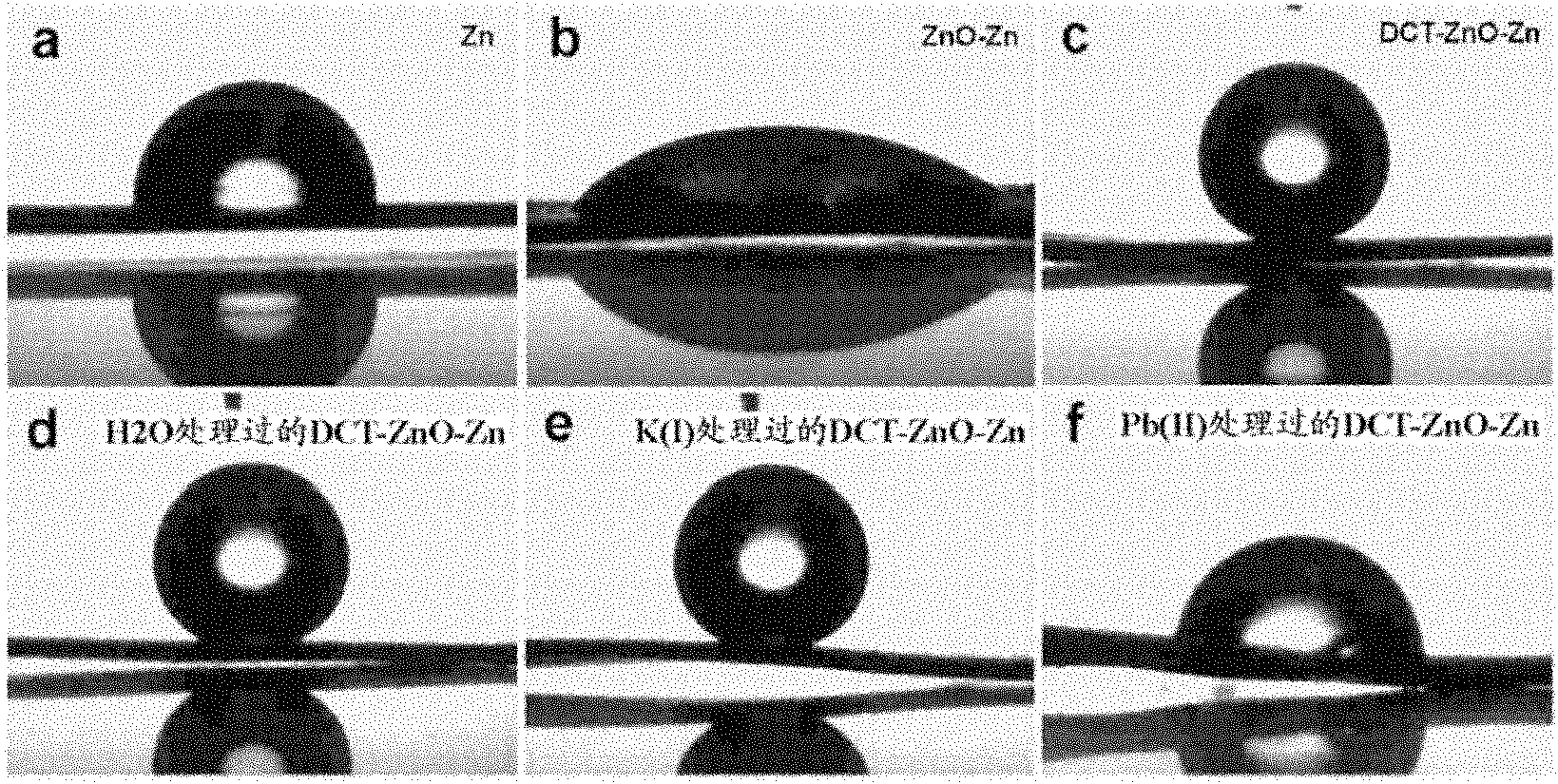Method and device for detecting heavy metal ion in water
A technology for heavy metal ions and detection materials, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as increasing the complexity of experimental operations, and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
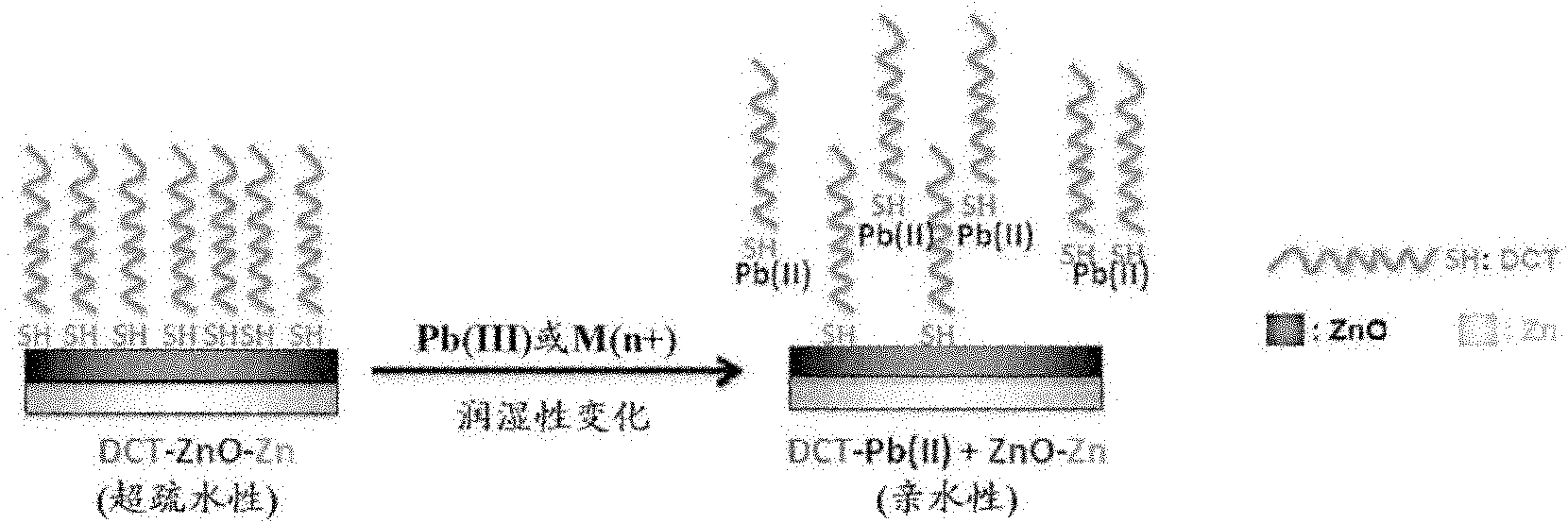
[0120] The preparation of the detection material of the present application may include: a first step, providing a material comprising a hydrophilic layer and an optional substrate (wherein the hydrophilic layer at least partially covers the optional substrate); second step, A hydrophobic layer that at least partially covers the hydrophilic layer is formed by the long-chain compound, wherein the long-chain compound is selected from long-chain thiols, long-chain aliphatic acids, and combinations thereof to obtain the detection material of the present application, wherein covering The region with the hydrophobic layer is called the detection region, and the initial contact angle between the surface of the detection region and water is greater than or equal to about 120°, preferably greater than or equal to about 150°.
[0121] In one embodiment, a material comprising a hydrophilic layer and a substrate is provided, wherein the hydrophilic layer at least partially covers the subst...
Embodiment 1
[0178] The superhydrophobic zinc flakes were prepared by the following steps: Zinc flakes were oxidized in 8% (v / v) N,N-dimethylacetamide-water solution at 65°C for 72h, taken out and cleaned with deionized water, and then placed at room temperature It is immersed in a 5mMDCT solution (the solvent is ethanol) for 24 hours, and self-assembled to obtain a superhydrophobic zinc sheet (DCT-ZnO-Zn). The entire surface of the superhydrophobic zinc sheet can be a detection area.
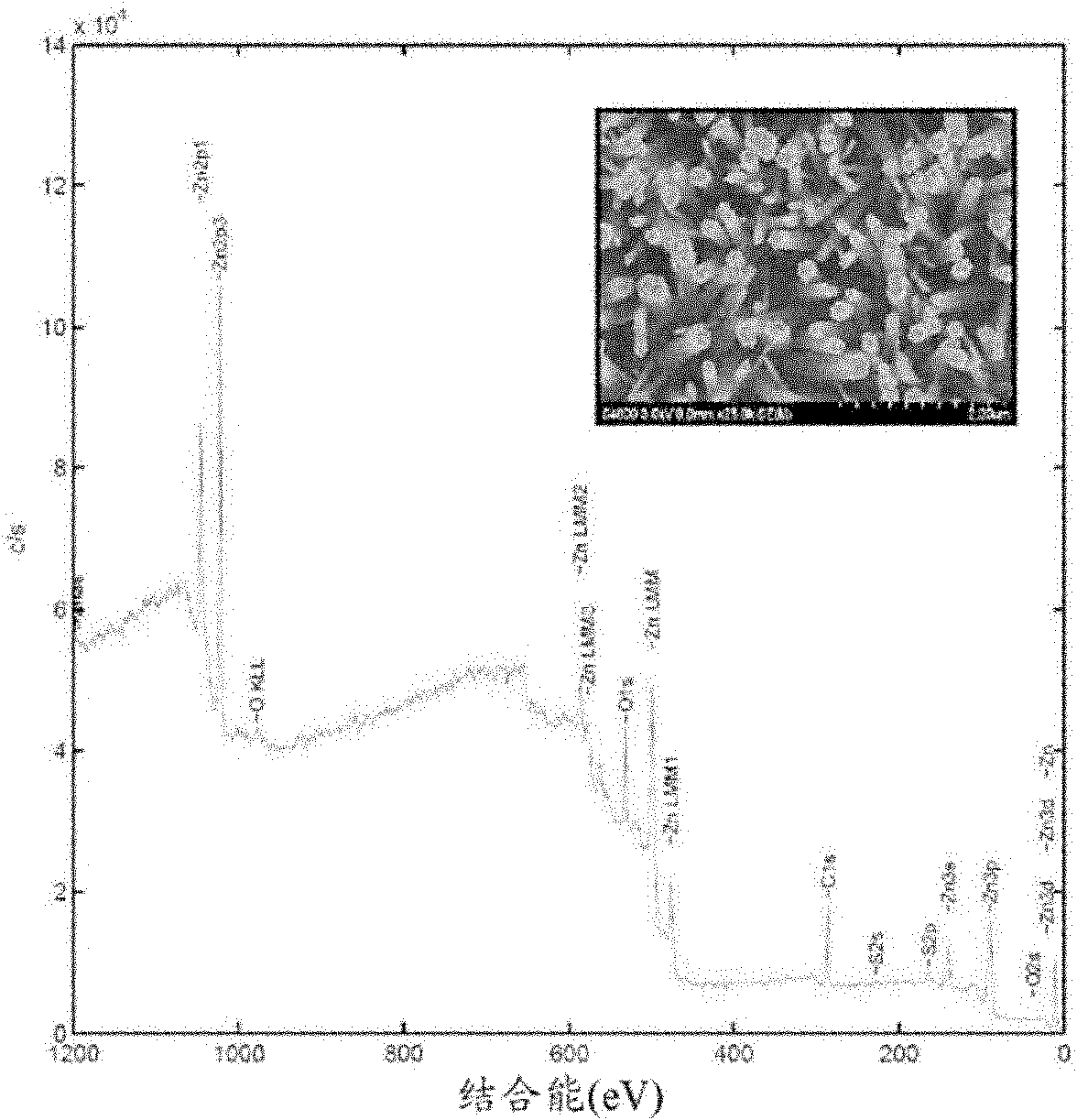
[0179] During the above preparation process, the obtained material can be analyzed by scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). figure 2 SEM images of the formed ZnO nanolayers and XPS spectra of superhydrophobic zinc flakes after oxidation of zinc flakes in 8% N,N-dimethylacetamide-water solution are described. As a result, the oxidized Zn surface appears as an array of highly oriented and closely packed hexagonal ZnO crystalline nanorods. After self-assembly of n-do...
Embodiment 2
[0188] As mentioned above, adding organic substances to the aqueous solution to be tested, and / or carrying out electrical treatment can shorten the detection time, lower the minimum detection limit and increase the detection speed.
[0189] In this example, the detection of heavy metal ions in aqueous solution using the superhydrophobic zinc sheet DCT-ZnO-Zn prepared in Example 1 was investigated under the condition of adding ethanol (EtOH) and / or energizing treatment.
[0190] In this example, 5% volume (based on the volume of the aqueous solution to be tested) of EtOH was added to the aqueous solution to be tested as a water-soluble solubilizer. The energization treatment can be performed as follows: the superhydrophobic zinc sheet acts as the working electrode, the Pt wire acts as the counter electrode and the Ag / AgCl electrode acts as the reference electrode, and a negative voltage (-3V) is applied to the superhydrophobic zinc sheet.
[0191] In addition, for better observ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com