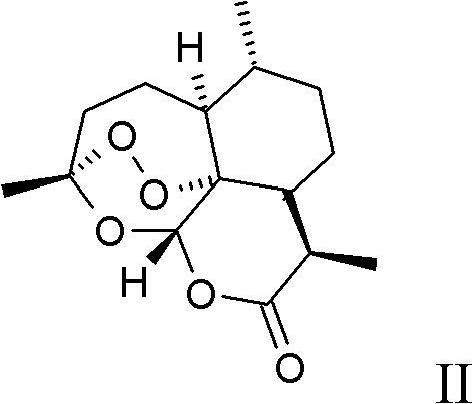
Process for the production of artemisinin intermediates
An in-situ preparation, diimine technology, applied in the preparation of carboxylate, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of trace catalyst poison sensitivity, high cost, low substrate and catalyst loading, etc. The effect of good product purity and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
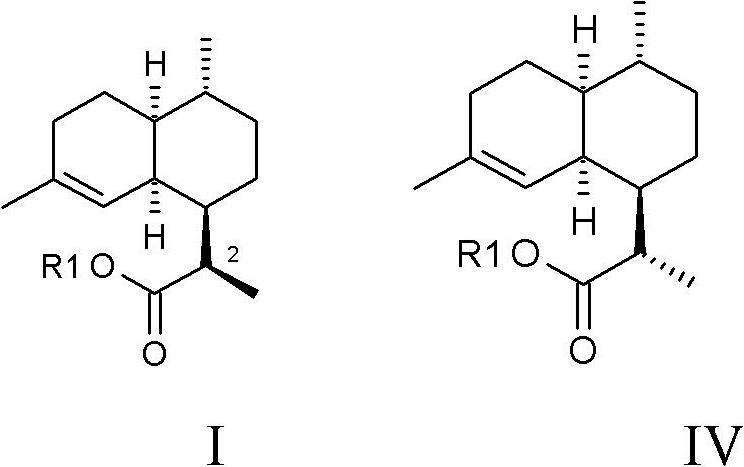
[0084] Example 1: Synthesis of dihydroartemisinic acid Ia in methanol at 65°C by generation of diimine from HOSA and sodium methoxide
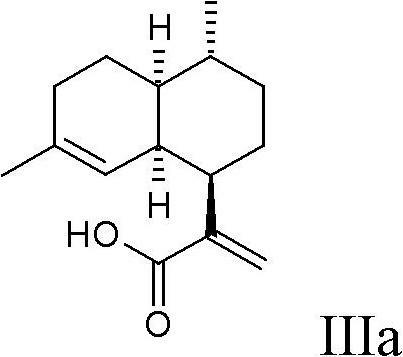
[0085] 0.248 g (0.001 mol) artemisinic acid IIIa was dissolved in 10 mL MeOH. Then 0.432 g (0.008 mol) sodium methoxide was added. The reaction mixture was heated to reflux (65° C.) and 0.628 g (0.005 mol) of hydroxylamine-O-sulfonic acid (HOSA) were added in portions. After complete addition, the reaction mixture was stirred at the same temperature for an additional 1 h until RP-HPLC analysis showed complete consumption of starting material. The reaction mixture was acidified to pH 2 with dilute aqueous hydrochloric acid. The product was extracted with MTBE, dried over magnesium sulfate and the solvent was evaporated to give 0.22 g (93%) of the title compound which crystallized on standing. pass 1 H-NMR and HPLC / MS analysis determined the diastereomer ratio in the unpurified product to be greater than 96:4, confirming the desired stereois...
Embodiment 2
[0095] Example 2: Synthesis of Dihydroartemisinic Acid Ia in Methanol at 40-50° C. by Generation of Diimine from Hydroxylamine and HOSA
[0096] 5.3 g (0.08 mol) of hydroxylamine (50% in water) and 15.1 g (0.12 mol) of HOSA (dissolved in 25 mL of water) were successively added to a solution of 4.69 g (0.02 mol) of artemisinic acid IIIa in 10 mL of MeOH, simultaneously with 5N NaOH aqueous solution kept the pH constant at pH 9. The temperature range is 40°C-50°C. After complete addition, the reaction mixture was stirred for an additional 1 hour until no change in pH was detectable. Complete consumption of artemisinic acid Ia was confirmed by RP-HPLC analysis. The reaction mixture was then acidified to pH 2 with dilute aqueous hydrochloric acid. The product was extracted with MTBE, dried over magnesium sulfate and the solvent was evaporated to give 4.8 g (100%) of the title compound which crystallized on standing. pass 1 H-NMR and LC / MS analysis detected a diastereomeric ra...
Embodiment 3
[0097] Example 3: Synthesis of dihydroartemisinic acid Ia by generation of diimine from hydroxylamine and HOSA / NaOH in methanol at temperatures between -5°C and 0°C
[0098] 2.34 g (0.01 mol) artemisinic acid IIIa was dissolved in 20 mL MeOH. Then 1.98 g (0.03 mol) of hydroxylamine (50% in water) and 5.65 g (0.045 mol) of HOSA (dissolved in 10 mL of water) were added successively while maintaining pH 9 with 32% aqueous NaOH. Adjust the temperature between -5°C and 0°C. After complete addition, the reaction mixture was stirred for an additional 1 hour until no change in pH was detectable. Complete consumption of artemisinic acid was confirmed by RP-HPLC analysis. The reaction mixture was then acidified to pH 2 with dilute aqueous hydrochloric acid. The product was extracted with MTBE, dried over magnesium sulfate and the solvent was evaporated to give 2.25 g (95%) of the title compound which crystallized on standing. pass 1 H-NMR and LC / MS analysis detected a diastereomer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com