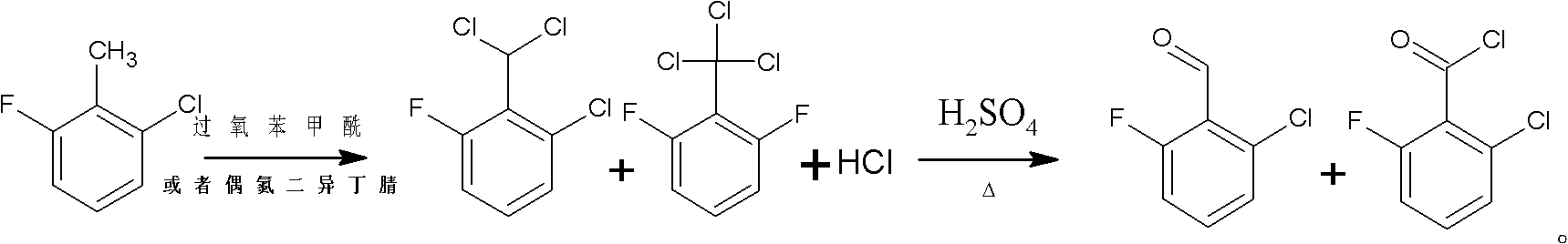
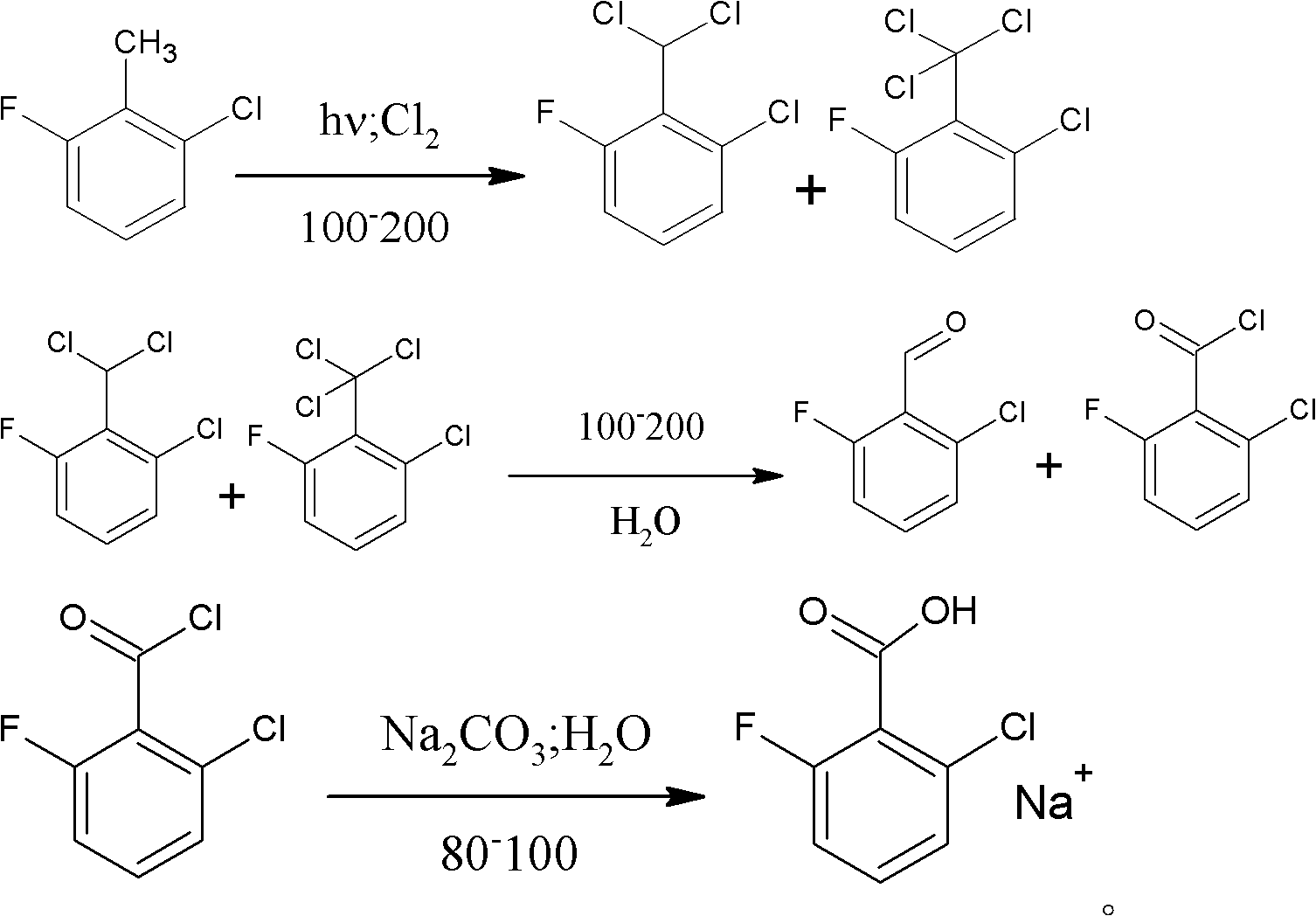
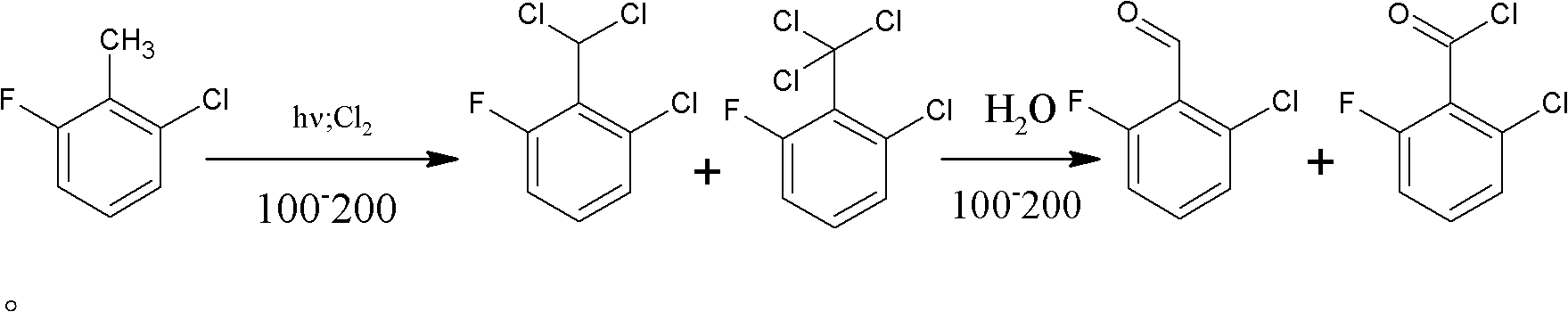
Method for preparing 2-chloro-6-fluorobenzaldehyde
A technology of fluorobenzaldehyde and fluorotoluene is applied in the field of preparation of 2-chloro-6-fluorobenzaldehyde, can solve the problems of complex reaction process, high cost, high risk and the like, and achieves good product quality, reduced risk, Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The concrete process that present embodiment prepares 2-chloro-6-fluorobenzaldehyde is as follows:
[0024] 1) add 250g 2-chloro-6-fluorotoluene and 0.5ml phosphorus trichloride in the 500ml four-necked glass reaction flask that reflux condenser, tail gas absorption device and thermometer are housed (add phosphorus trichloride in this step and can To improve product quality), chlorine gas is introduced under the condition of 180°C under the irradiation of a metal halide lamp.
[0025] 2) detect the content of 2-chloro-6-fluorobenzyl chloride in the reaction flask liquid phase with gas chromatography, when detecting that the content of 2-chloro-6-fluorochlorobenzyl is less than 0.5%, stop logical chlorine, simultaneously to reaction flask Nitrogen was introduced into the bottle to remove unreacted chlorine in the bottle.
[0026] 3) Add 0.5g of iron-based solid superacid SO to the liquid phase of the reaction flask 4 2- / Fe 3 o 4 , under the condition of 180 ° C, 37...
Embodiment 2
[0029] 1) Add 250g of 2-chloro-6-fluorotoluene to a 500ml four-necked glass reaction flask equipped with a reflux condenser, tail gas absorption device and thermometer, and feed chlorine gas under the irradiation of a metal halide lamp at 150°C.
[0030] 2) detect the content of 2-chloro-6-fluorobenzyl chloride in the reaction flask liquid phase with gas chromatography, when detecting that the content of 2-chloro-6-fluorochlorobenzyl is less than 0.5%, stop logical chlorine, simultaneously to reaction flask Nitrogen was introduced into the bottle to remove unreacted chlorine in the bottle.
[0031] 3) Add 1 g of iron-based solid superacid SO to the liquid phase of the reaction flask 4 2- / Fe 3 o 4 , under the condition of 150°C, 40g of water was slowly and uniformly dropped into the reaction bottle within 3 hours, and kept warm for 4 hours, and the conversion of 2-chloro-6-fluorodichlorobenzyl and 2-chloro-6-fluorotrichlorobenzyl was detected by gas chromatography Complete...
Embodiment 3
[0034] This embodiment is basically the same as Embodiment 2, the difference is: (1) in the 1st) step, the light temperature is 120°C; (2) in the 3rd) step, the temperature when dripping water is 120°C; The temperature during potassium carbonate aqueous solution is 80 ℃; The pH value of adjusting mixed solution is 10; The holding time is 4.2 hours;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com