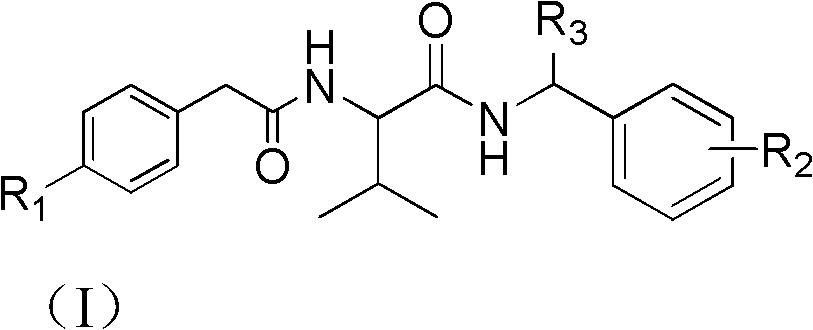
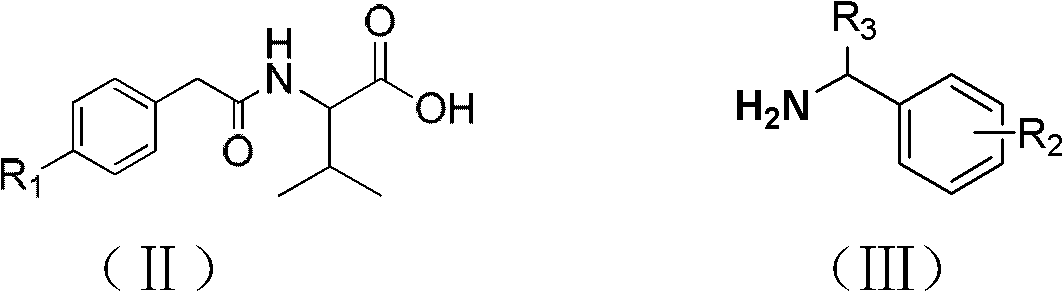
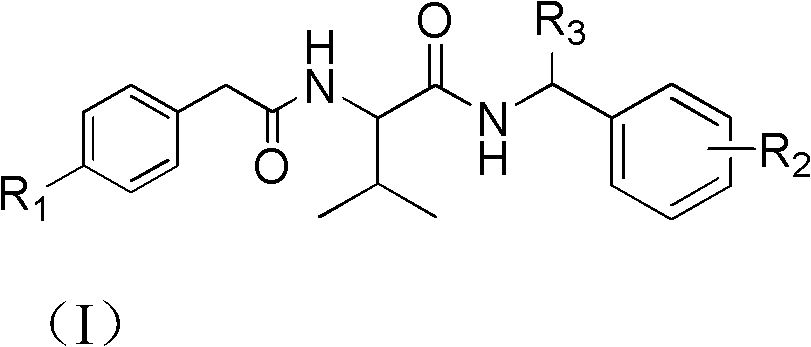
Bisamide type compound and application thereof
A compound and amide technology, applied in the field of preparation of bisamide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Taking the preparation of IIa as an example, oxalyl chloride (2.86g, 22.5mmol) was added to a 50ml single-necked bottle, and phenylacetic acid IVa (2.04g, 15mmol dissolved in 20ml of dichloromethane) was added dropwise in an ice-water bath. Into the reaction system, after 30 minutes of dripping, react at room temperature for 24 hours, then spin out the solvent and excess oxalyl chloride in the reaction solution, and the remaining liquid is for subsequent use. Dissolve valine (2.11g, 18mmol) in 36ml of sodium hydroxide (1.44 g, 36mmol) solution, then slowly drop in the above-mentioned prepared acid chloride residual liquid, drop it in 4h, react at room temperature for 10h, adjust the pH of the reaction solution to be about 1, solids are precipitated, suction filtered, and the filter cake is dried to obtain White powder 2.9g g. The preparation methods of IIb and IIc are similar, except that R in the formula IV 1 Respectively for the corresponding substituent groups, that...
Embodiment 1
[0034] Embodiment 1: 3-Methyl-N-(1-phenylethyl)-2-(2-(4-propargyloxyphenyl) acetamido) butyramide: Synthesis of (I-1)
[0035] 2-(2-(4-propargyloxyphenyl)acetamido)-3-methylbutanoic acid (2.5mmol) was added to 10ml of dried dichloromethane, stirred evenly under ice-salt bath conditions, Control the temperature at -15~-10°C, add triethylamine (2.75mmol), then dropwise add isobutyl chloroformate (2.5mmol), stir in an ice-water bath for 1h, and still under this condition, add dropwise 1-benzene Ethylamine (2.75mmol dissolved in 10ml of dichloromethane), dripped for 15min, reacted at room temperature for 1h, washed with 10wt% dilute hydrochloric acid, spin-dried, the crude product was recrystallized with ethanol, and suction filtered to obtain a khaki solid with a yield of 67 %, 1 H NMR (400MHz, CDCl 3 ): δ7.36-7.25 (m, 5H, Ar-H), 7.20 (d, J=8.0Hz, 2H, Ar-H), 6.95 (d, J=8.4Hz, 2H, Ar-H), 6.64 (s, 1H, NH), 6.32 (s, 1H, NH), 5.07-5.04 (m, 1H, NH CH CH 3 ), 4.69 (s, 2H, O CH 2...
Embodiment 2
[0036] Example 2: 3-methyl-2-(2-(4-propargyloxyphenyl) acetamido)-N-(1-(p-tolyl) ethyl) butyramide: (I-2) Synthesis
[0037] 2-(2-(4-propargyloxyphenyl)acetamido)-3-methylbutanoic acid (2.5mmol) was added to 10ml of dried dichloromethane, stirred evenly under ice-salt bath conditions, Control the temperature at -15~-10°C, add triethylamine (2.75mmol), then dropwise add isobutyl chloroformate (2.5mmol), stir in an ice-water bath for 1h, still under this condition, add dropwise 1-( 4-Methylphenyl)ethylamine (2.75mmol dissolved in 10ml of dichloromethane), after 15 minutes of dripping, reacted at room temperature for 1h, washed with 10wt% dilute hydrochloric acid, spin-dried, the crude product was recrystallized with ethanol, and filtered to obtain soil Yellow solid, 50% yield, 1 H NMR (400MHz, CDCl 3 ): δ7.22-7.15 (m, 6H, Ar-H), 6.96 (t, J=8.4Hz, 2H, Ar-H), 6.24 (dd, J 1 =8.4Hz,J 2 = 17.2Hz, 1H, NH), 6.07(dd, J 1 =8.0Hz,J 2 =20.8Hz, 1H, NH), 5.06-4.99 (m, 1H, NH CH CH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com