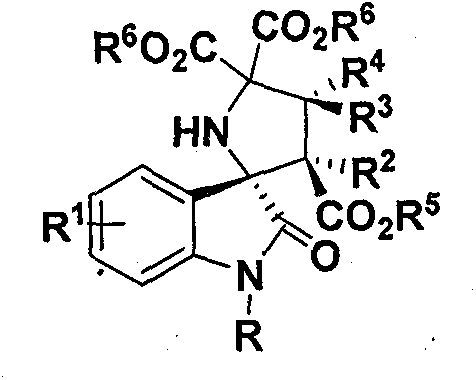
Chiral spiro(pyrrolidine-3, 2'-oxindole)compound and synthesis method thereof
A technology of tetrahydropyrrole and indole spirocycles, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of limited substrate scope, limited methods, uneconomical and easy-to-obtain chiral raw materials, etc., and achieve operational Simple, mild reaction conditions, economical and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
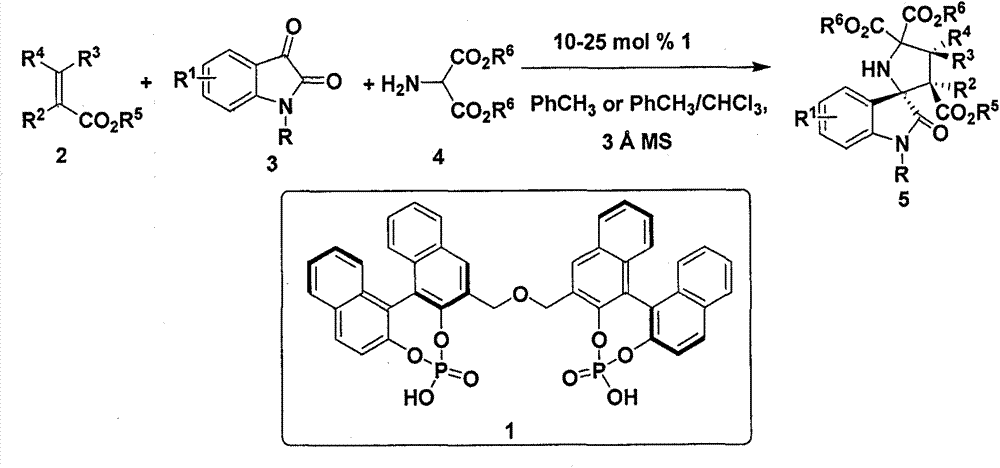
[0056] The (R)-type chiral phosphoric acid catalyst represented by 0.12mmol of N-methylisatin, 0.1mmol of 2-aminomalonate, 0.01mmol of formula 1, 100mg Molecular sieves (purchased from Tianjin Alpha Aisha Company) were put into a 10 mL glass reaction test tube, 0.5 mL of toluene was added, and stirred at room temperature for 15 minutes (600 rpm / min), then 0.5 mmol of dimethyl maleate and 0.5 mL of Toluene, react at 25°C for 36 hours.
[0057] Then, 4 mL of ethyl acetate was added to the test tube containing the reaction mixture to dilute the reaction mixture. Spread a layer of thin-layer chromatography silica gel H on a glass funnel plugged with cotton, pump the silica gel tightly with a water pump, then pour the reaction mixture in the test tube into the funnel, and filter out molecular sieves, including molecular sieves. A layer of chromatographic silica gel remains in the funnel as a filter cake. Next, wash the suction filter cake three times with 15 mL of ethyl acetate,...
Embodiment 2
[0062] Adopt the method identical with embodiment 1, wherein: the isatin that adopts is N-benzyl isatin, adds thin-layer chromatography silica gel H in common glass column, pressurizes column chromatography with nitrogen (column length 15 centimetres, flow velocity 3 drops / second), the eluent was petroleum ether: ethyl acetate 5:1 (volume ratio), and the product 5b was obtained with a yield of 88%, dr>99:1, ee 93%.
[0063] Characterization data of compound 5b:
[0064]
[0065] (2'S, 3'R, 4'S)-5', 5'-Diethyl3', 4'-dimethyl1-benzyl-2-oxospiro[indoline-3, 2'-pyrrolidine]-3', 4', 5′,5′-tetracarboxylate (5b): white solid; m.p.99-101℃; [α] D 20 =-44.3 (c 2.98, CHCl 3 ); 1 H-NMR (CDCl 3 , 400MHz) δ (ppm): 7.69 (dd, J 1 =7.6Hz,J 2 =0.9Hz, 1H, ArH), 7.31-7.22(m, 5H, ArH), 7.15(td, J 1 =7.8Hz,J 2 =1.2Hz, 1H, ArH), 6.99(td, J 1 =7.6Hz,J 2 =1.0Hz, 1H, ArH), 6.61(d, J=7.7Hz, 1H, ArH), 5.20(d, J=15.7Hz, 1H, CH), 4.56(d, J=8.1Hz, 1H, CH) , 4.51 (d, J=15.7Hz, 1H, CH), 4.44-4....
Embodiment 3
[0067] Adopt the method identical with embodiment 1, wherein: the isatin that adopts is N-phenyl isatin, adds thin-layer chromatography silica gel H in common glass column, pressurizes column chromatography with nitrogen (column length 15 centimetres, flow velocity 3 drops / second), the eluent is petroleum ether: ethyl acetate 5:1 (volume ratio), and the product 5c is obtained with a yield of 90%, dr>99:1, ee 80%.
[0068] Characterization data of compound 5c:
[0069]
[0070] (2'S, 3'R, 4'S)-5', 5'-Diethyl 3', 4'-dimethyl2-oxo-1-phenylspiro[indoline-3, 2'-pyrrolidine]-3', 4' , 5′,5′-tetracarboxyIate (5c): white solid; m.p.129-131℃; [α] D 20 =-67.3 (c 0.944, CHCl 3 ); 1 H-NMR (CDCl 3 , 400MHz) δ (ppm): 7.75 (dd, J 1 =7.6Hz,J 2 =0.9Hz, 1H, ArH), 7.54-7.47(m, 2H, ArH), 7.43-7.36(m, 3H, ArH), 7.21(td, J 1 =7.8Hz,J 2 =1.3Hz, 1H, ArH), 7.07(td, J 1 =7.6Hz,J 2 =1.0Hz, 1H, ArH), 6.72(d, J=7.7Hz, 1H, ArH), 4.60(d, J=8.1Hz, 1H, CH), 4.40-4.22(m, 4H, 2CH 2 ), 4.16 (d, J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com