Preparation method of lithium ion anode material coated with lithium ion activating oxide V2O5
A technology of active oxides and cathode materials, which is applied in the field of preparation of lithium-ion battery materials, can solve the problems of affecting the rate performance of materials, not having lithium ion channels, hindering lithium ion transmission, etc., achieving excellent physical and electrochemical properties, Improved air storage performance and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
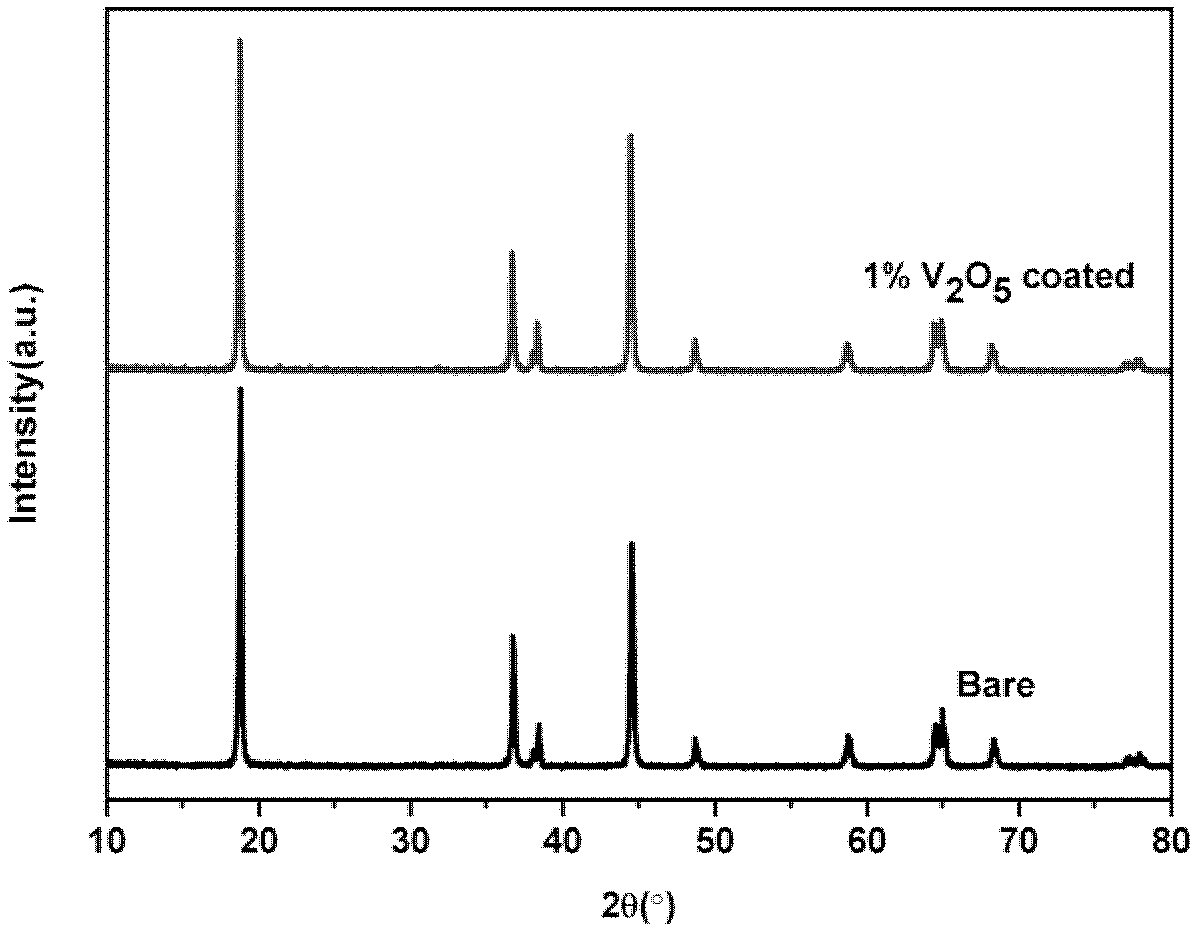
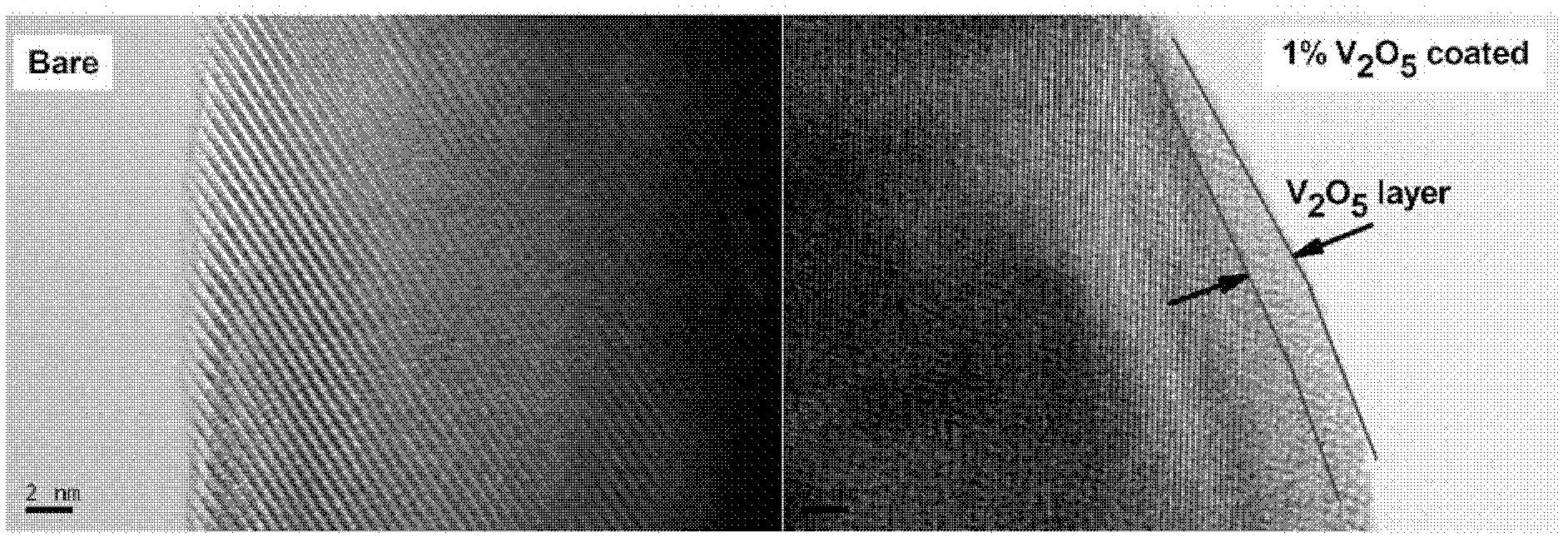
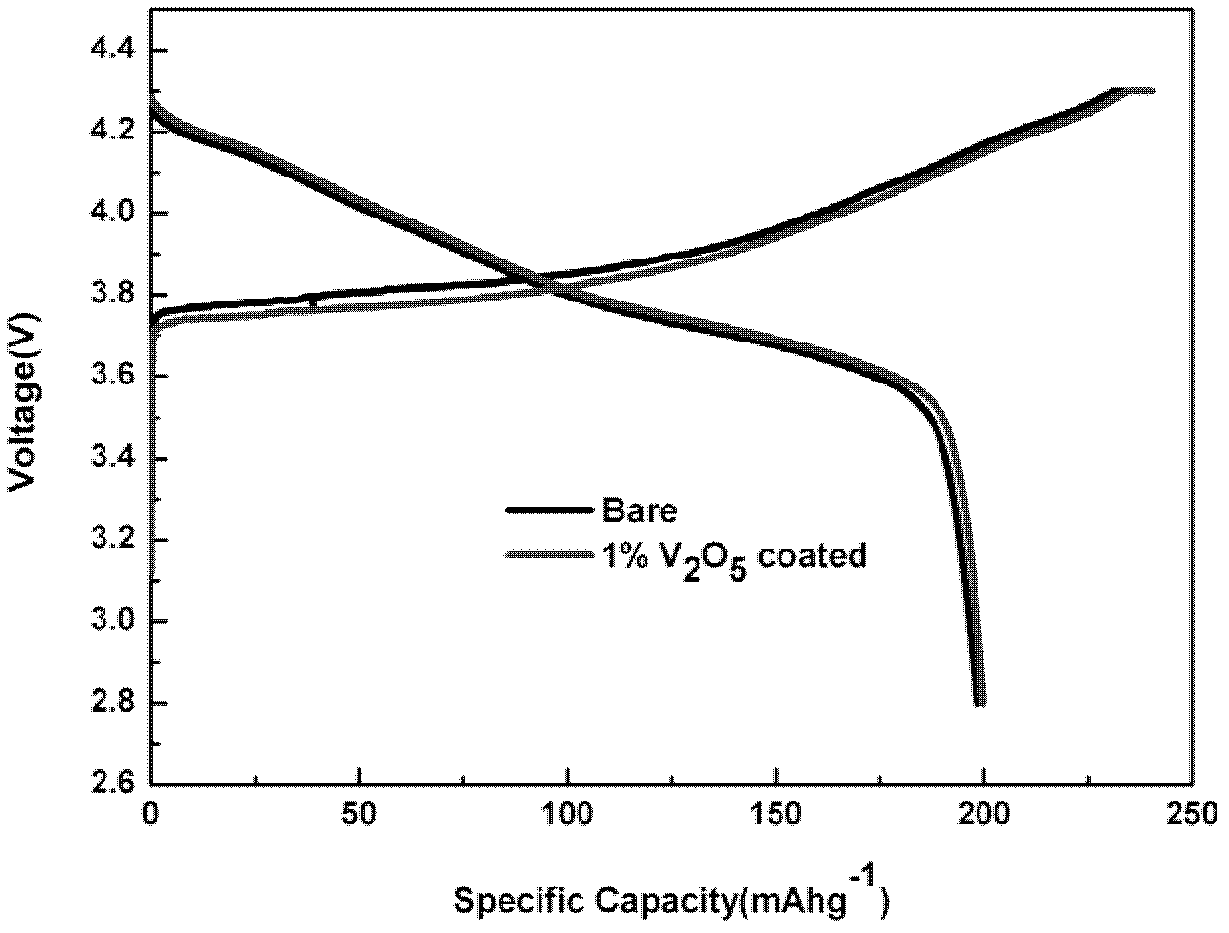
[0032] Will NH 4 VO 3 Dissolve in deionized water at 60°C, under constant stirring, at V 2 o 5 The coating amount is calculated as 1%, and LiNi is added to it 0.8 co 0.1 mn 0.1 o 2 material and keep the temperature constant. After evaporating water in a 60°C oil bath, heat treatment at 400°C for 6 hours, and cool naturally to obtain the coating material. For its physical and chemical properties, see figure 1 , figure 2 , image 3 with Figure 4 . XRD shows no V 2 o 5 It can be seen from the TEM image that there is a uniform layer of V with a thickness of about 2nm on the surface of the material. 2 o 5 layer, while it can be seen that V 2 o 5 layer with LiNi 0.8 co 0.1 mn 0.1 o 2 There is no clear boundary between the host material and this is because the V 2 o 5 Will be with LiNi 0.8 co 0.1 mn 0.1 o 2 Reaction of Li residues on the material surface. The resulting product was assembled into a button battery to measure its charge and discharge capaci...
Embodiment 2
[0034] Will NH 4 VO 3 Dissolve in 5% ammonia water, under constant stirring, at V 2 o 5 The coating mass ratio is calculated as 1%, and Ni is added to it 0.8 co 0.1 mn 0.1 (OH) 2 materials, stir and disperse evenly, and evaporate the water in an 80°C oil bath, dry the powder at 120°C and mix with Li 2 CO 3 Mix evenly, keep warm at 500° C. and 750° C. for 5 hours and 15 hours respectively under an oxygen atmosphere, and cool naturally to obtain a coating material. The resulting product was assembled into a button battery to measure its charge and discharge capacity and rate performance, and charge and discharge were performed at different rates. From the 2C rate cycle performance diagram of the material at room temperature, it can be seen that the capacity of the coated material does not attenuate after 100 2C cycles.
Embodiment 4
[0036] will V 2 o 5 Dissolve in 3% hydrogen peroxide, under constant stirring, at V 2 o 5 The coating amount is calculated as 1%, and LiNi is added to it 0.8 co 0.1 mn 0.1 o 2 Materials, strong stirring and ultrasonic dispersion make the system evenly mixed. The coating material precursor was obtained by spray drying, kept at 400° C. for 5 hours in an air atmosphere, and cooled naturally to obtain the coating material. The resulting product was assembled into a button battery to measure its charge and discharge capacity and rate performance, and charge and discharge were performed at different rates. The results are shown in Table 1. In order to compare the storage stability of the material in the air before and after the material coating, the two materials were exposed to the air, and the H in the air adsorbed by the material was determined by titration method. 2 O and CO 2 content.
[0037] Table 1 Electrochemical properties of materials before and after coating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com