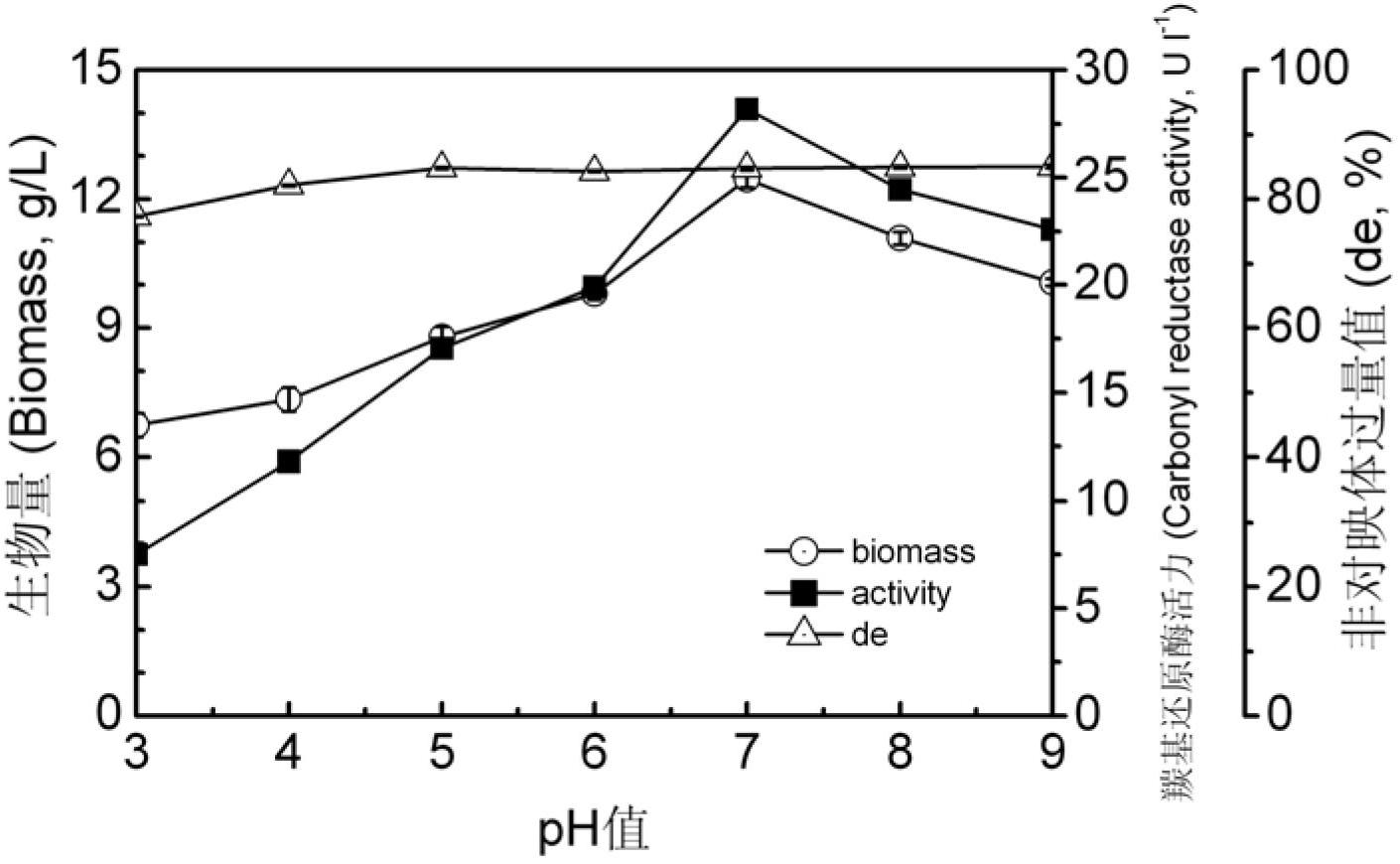
6- cyano-(3r, 5r)-dyhydroxyl hexanoic acid tert-butyl ester prepared by biological catalysis, and bacterial strain thereof
A technology of tert-butyl hydroxycaproate and strains, which is applied in the field of biocatalytic preparation of tert-butyl 6-cyano-(3R,5R)-dihydroxyhexanoate and strains, and can solve the problems of insufficient diastereomeric induction, diastereomeric Problems such as low de value of tert-butyl hydroxycaproate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Screening microbial strains with catalytic (R)-6-cyano-5-hydroxyl-3-oxoylhexanoic acid tert-butyl ester reduction activity
[0041] Collect soil samples from all over the country, take 1.0g soil samples and disperse them into 10.0mL, 0.85% normal saline solution, mix well; take 2.0mL bacterial suspension and inoculate to 28mL containing 100mM (R)-6-cyano-5- Hydroxy-3-oxohexanoic acid tert-butyl ester enrichment medium (preparation: 50.0g glucose, 100.0g soybean sprouts boiled for 30 minutes, added 22.8g (R)-6-cyano-5-hydroxy-3-oxohexanoic acid tert-butyl ester, add water to make up to 1.0L, pH is natural); at 28°C and 150rpm, culture on a shaking table until the culture medium becomes turbid; transfer to fresh sterile In the enrichment medium (the composition is the same as before), the shaking culture was continued for 4 days under the conditions of 30° C. and 150 rpm, and the second batch of enrichment culture was obtained.
[0042] The second batch of enr...
Embodiment 2
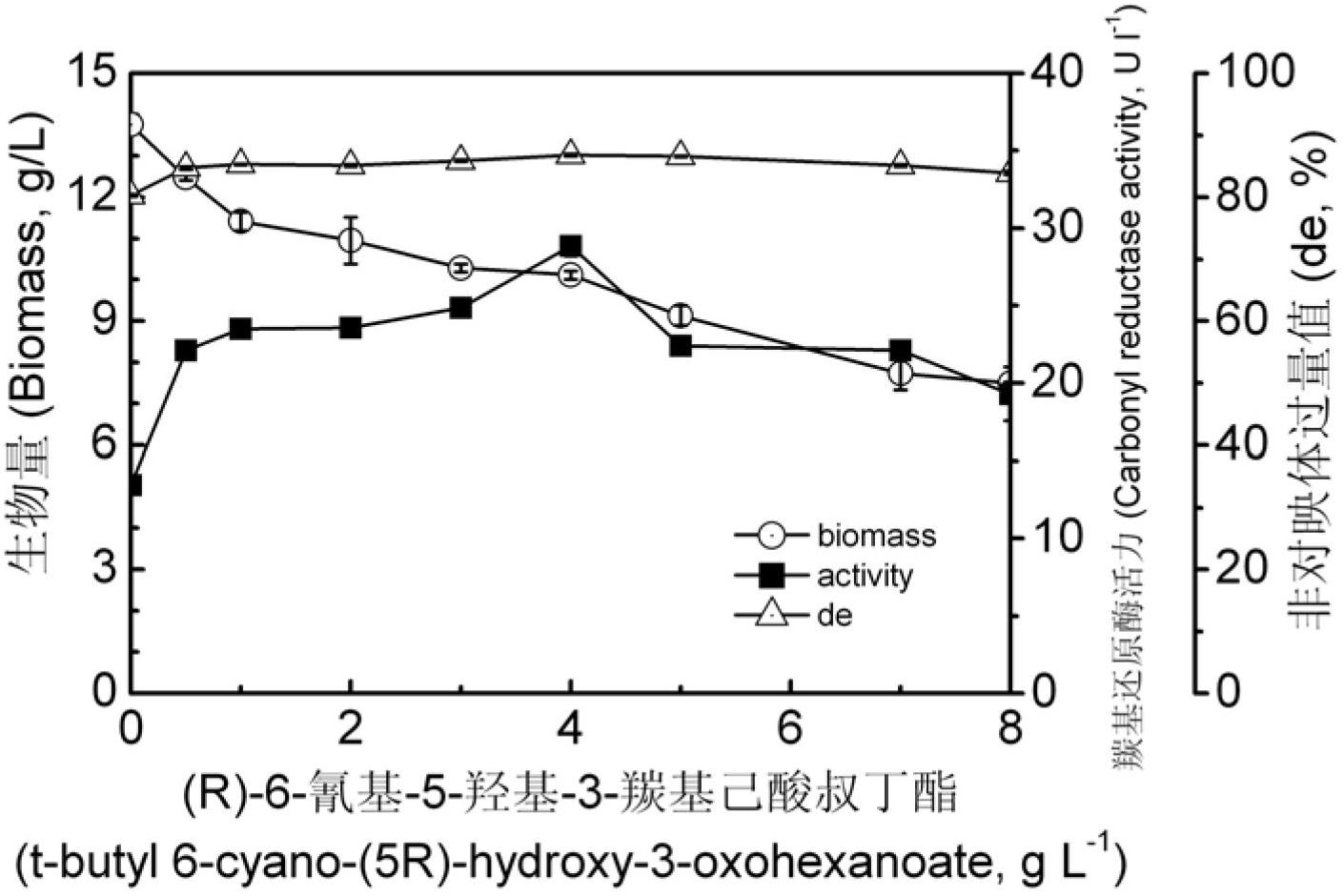
[0045] Example 2: Influence of (R)-6-cyano-5-hydroxy-3-oxoylhexanoic acid tert-butyl concentration on Pichia guilliermondii X25 carbonyl reductase volume enzyme activity and diastereoselectivity in fermentation medium
[0046] For the Pichia guilliermondii X25 bacterial classification that will be preserved on the test tube slant, pick an inoculation loop thallus and inoculate to contain 0g / L,0.5g / L,1.0g / L,2.0g / L,3.0g / L,4.0g / L In the sterile fermentation medium of L, 5.0g / L, 7.0g / L, 8.0g / L (R)-6-cyano-5-hydroxy-3-oxoylhexanoic acid tert-butyl ester, other fermentation medium The component formula is as follows: yeast extract 25.0g / L, glucose 25.0g / L, (NH 4 ) 2 HPO 4 2.5g / L, KH 2 PO 42.5g / L, MgSO 4 30mg / L, NaCl 1.0g / L, solvent is water, pH 6.0. Cultivate at 28°C, 150rpm for 2 days, and collect the fermentation broth.
[0047] Pipette 20 mL of fermentation broth, centrifuge at 12,000 rpm for 8 minutes, discard the supernatant, collect the thalli, wash the thalline three ...
Embodiment 3
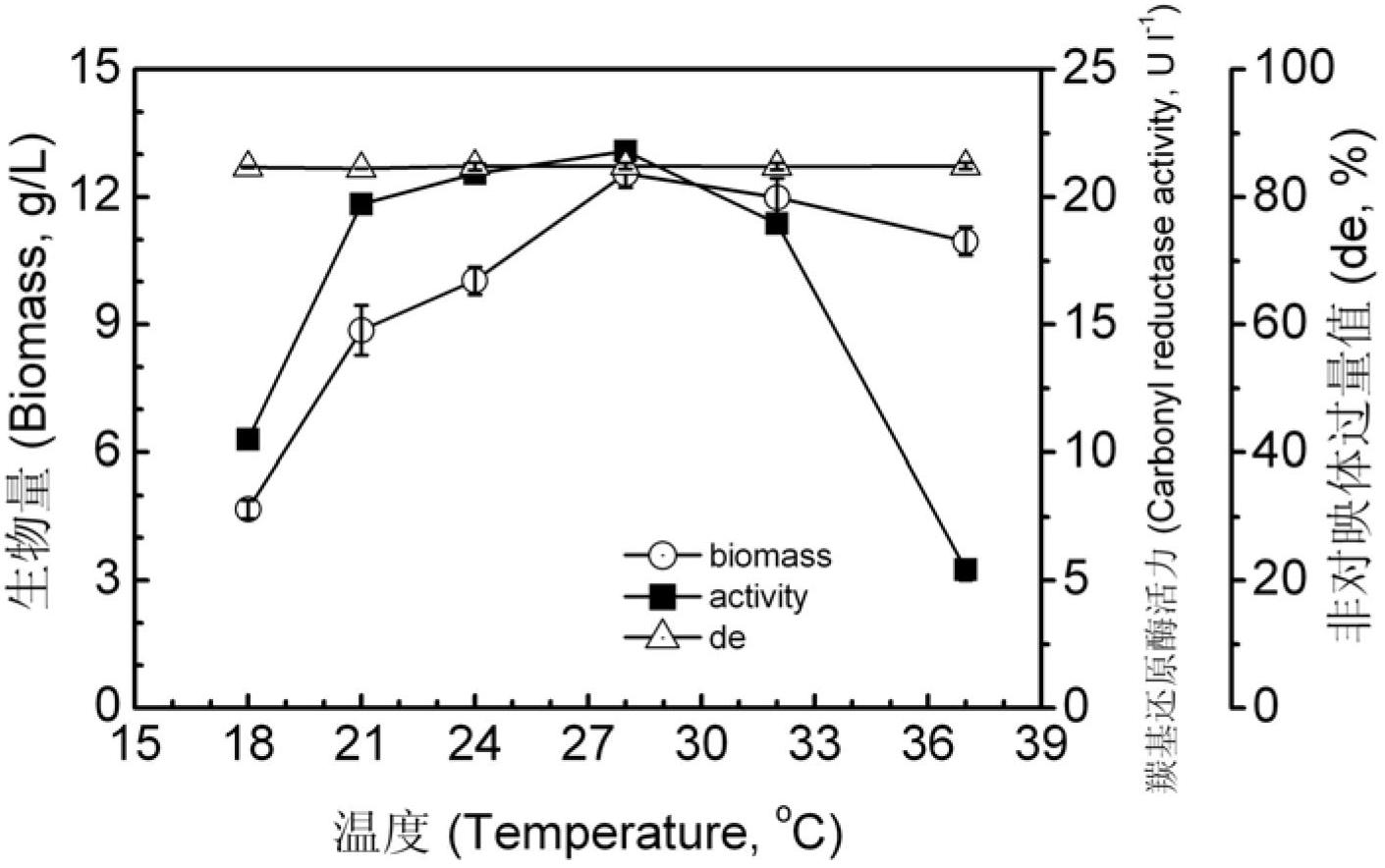
[0048] Embodiment 3: The impact of culture temperature on volume enzyme activity and diastereoselectivity of Pichia guilliermondii X25 carbonyl reductase
[0049] To the Pichia guilliermondii X25 bacterial classification that will be preserved on the test tube slant, pick an inoculation loop thalline and inoculate to the non-butyl ester containing 4.0g / L (R)-6-cyano-5-hydroxyl-3-carbonylhexanoate In the bacterial culture medium, here (R)-6-cyano-5-hydroxyl-3-oxoylhexanoic acid tert-butyl plays the role of inducer, and the other component formulations of the fermentation medium are as follows: yeast extract 25.0g / L, Glucose 25.0g / L, (NH 4 ) 2 HPO 4 2.5g / L, KH 2 PO 4 2.5g / L, MgSO 4 30mg / L, NaCl 1.0g / L, solvent is water, pH7.0, cultured at 18°C, 21°C, 24°C, 28°C, 32°C, 34°C, 37°C, 150rpm for 2 days, Collect the broth.
[0050] Pipette 20 mL of fermentation broth, centrifuge at 12,000 rpm for 8 minutes, discard the supernatant, collect the thalli, wash the thalline three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com