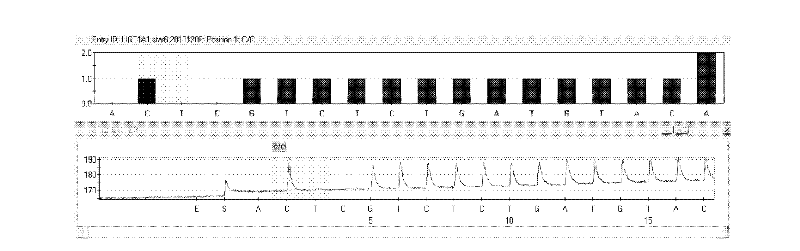
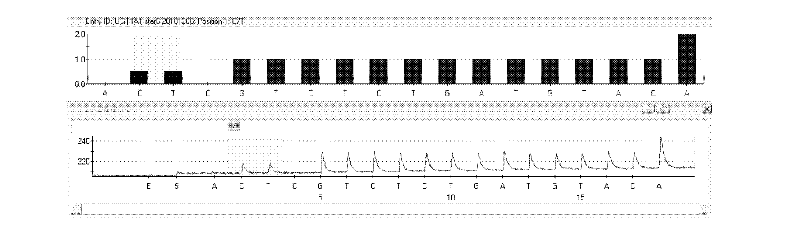
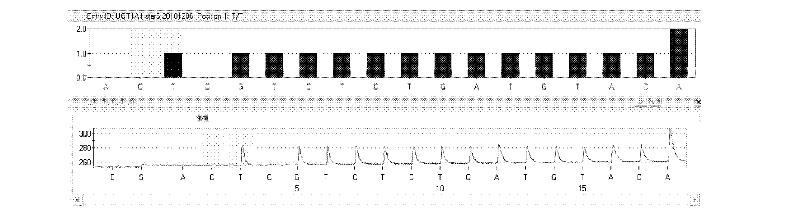
Kit and method for detecting gene polymorphism of irinotecan personalized medicine by pyrophosphoric acid sequencing method
A pyrosequencing method and gene polymorphism technology, applied in the field of molecular biology, can solve the problems of increased risk of toxic and side effects of irinotecan, and achieve the effects of simple operation and small sample size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] UGT1A1*6 (rs4148323) upstream primer: 5′-GAA ATA GTT GTC CTA GCA CC-3′ (SEQ ID NO.3);
[0034] UGT1A1*28 (rs3064744) upstream primer 5'-ACA GCT TTT TAT AGT CAC GTGA-3' (SEQ ID NO.4);
[0035] UGT1A1*6 (rs4148323) downstream primer: 5′-CTT CAA GGT GTA AAA TGC TC-3′ (SEQ ID NO.5);
[0036] UGT1A1*28 (rs3064744) downstream primer 5'-TGC TCA GCC AGT GGC TGC CATCCA-3' (SEQ ID NO.6);
[0037] UGT1A1*6 (rs4148323) sequencing primer: 5′-TCA AGG TGT AAA ATG CTC-3′ (SEQ ID NO.7);
[0038] UGT1A1*28 (rs3064744) sequencing primer: 5'-CGC CCT CTC CTA CTT AT-3' (SEQ ID NO.8);
[0039] 1. DNA extraction
[0040] 1.1 The preparation and inspection of reagent materials before the experiment are as follows:
[0041] (1) Check the shelf life of the kit and ensure that ethanol has been added to Wash Buffer 1 and 2, and tick the corresponding mark on the bottle; (2) Isopropanol (if not available, it can be replaced by absolute ethanol) and 75% ethanol ; (3) 1.5mL Eppendorf tubes and vari...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com