Selectively Split Whole Cell Vaccines
A whole-cell, cell-based technology, applied in the fields of immunology, bacteriology, and molecular genetics, can solve problems such as immunogenicity exploration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1. Antigen preparation
[0103] Strain Rx1E in which the pneumolysin gene was replaced by a detoxified mutant PdT was provided to us by James Paton (University of Adelaide, Australia). Using the strategy described previously, the entire lytA genomic coding region was replaced with a Janus expression cassette tagged with the kanamycin resistance gene rpsL (Sung et al., Appl. Environ. Microbio. 139 (2006); van Ginkel et al., J. Immunol. , 165, 4778 (2000)). Briefly, three PCR amplification products were generated: (i) with primers LAD1 (CAAGGTATCCATCATTCC) (SEQ ID NO: 1) and LAD2 (CGC GGATCC The 1 kb fragment upstream of the lytA genomic region amplified by ACAGTAGAGCCAGATGGC (SEQ ID NO: 2); the BamHI site is underlined); (ii) with primer LAD3 (TTT GGGCCC GTTGCACGCCGACTTGAGG (SEQ ID NO:3); ApaI site is underlined) and LAD4 (CTTTGCTTCTCAGAATCTAGG) (SEQ ID NO:4) amplified 800bp fragment downstream of lytA; and (iii) primers DAM351 (with ApaI site) and DAM406 ( ...
Embodiment 2
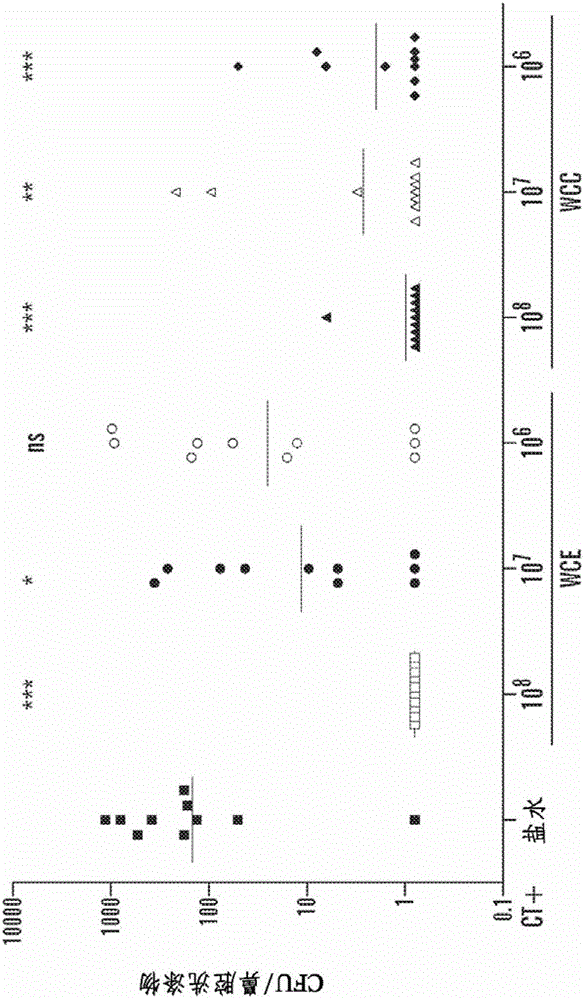
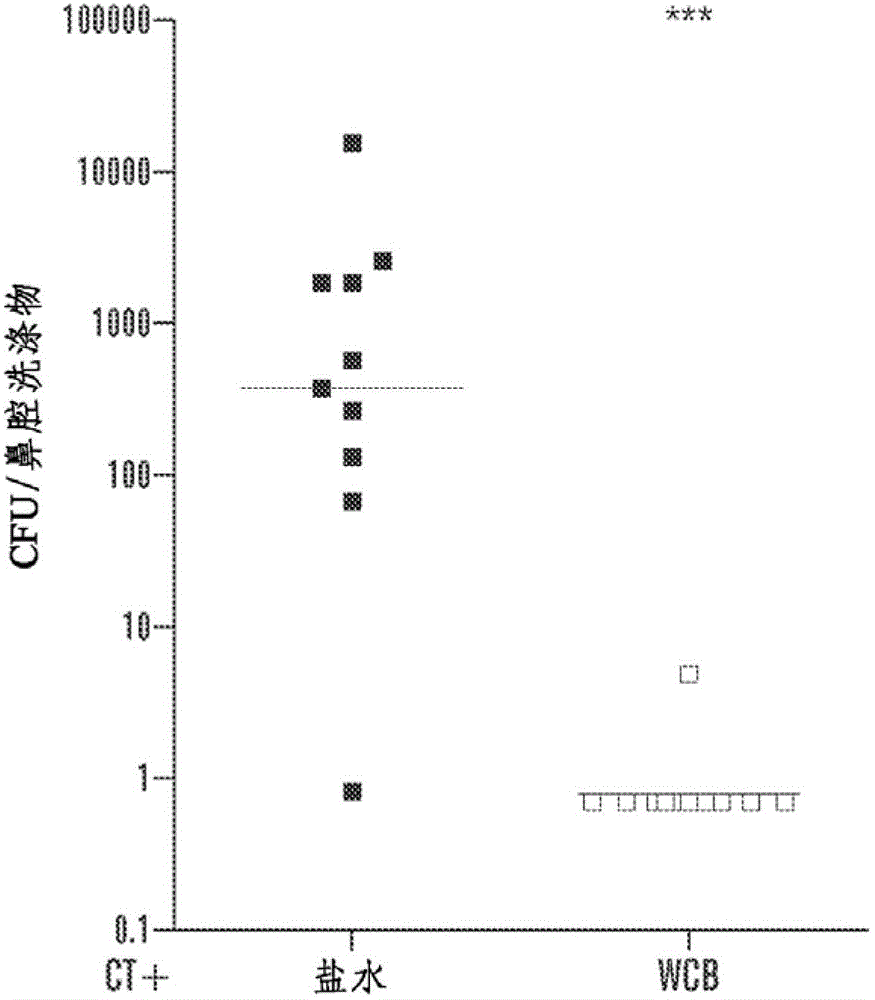
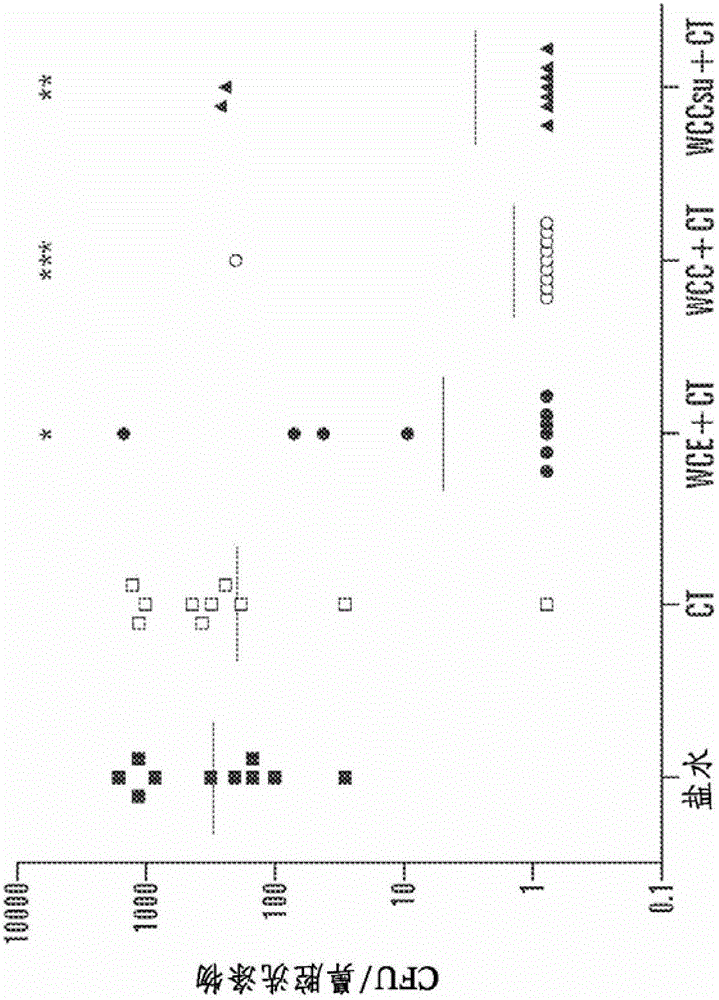
[0108] Example 2. Immunization and challenge of mice
[0109] C57BL / 6J mice (Jackson Laboratories, Bar Harbor, Maine) were used in all experiments. Age 4-6 weeks at the time of first immunization. Intranasal (i.n) immunization (a step that does not place the immunogen into the lungs) by atraumatically instilling 10 μL of saline, adjuvant alone, or adjuvant mixed with the specific antigen into non-anesthetized mice ; a second immunization was performed 1 week later. respectively by mixing with 1%NaHCO 3 Oral or sublingual immunizations were performed by placing 30 μL of the vaccine mixed with 30% sucrose on the oral surface or by placing 5 μL of the vaccine in the same diluent under the tongue. Oral or sublingual immunizations were given three times a week, whereas intranasal immunizations were given only twice.
[0110] WCC was used for transdermal immunoassay (TCI) experiments. WCCs were rehydrated in water containing 0.1% Zwittergent3-14 (Calbiochem, Gibbstown, NJ) and ...
Embodiment 3
[0114] Example 3. Rabbit Immunization and Toxicology Studies
[0115] All rabbit immunizations were performed at MPI (Mattawan, MI). On days 1, 15, 29 and 43, female New Zealand White rabbits in groups of three were given 0.5 ml of the following injections by intramuscular injection: saline; Al(OH alone ) 3 (contains 0.6 mg Al); doses of 50 μg, 500 μg or 5000 μg of Al(OH) 3 Adsorbed WCB or whole-cell diphtheria-tetanus-pertussis whole-cell (DTwP) vaccine (clinical product from Institute Butantan). Sera were obtained before each immunization and at the moribund exsanguination on day 45 and sent frozen to Children's Hospital Boston for antibody determination. For all animals, observations were made periodically for morbidity, mortality, clinical signs, body temperature, and food and water consumption. Dermal irritation scores were evaluated before each administration, and after each administration (except the last administration) were evaluated daily for three consecutive da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com