Eye-purposed in-vivo gel preparation prepared from pH (Potential Of Hydrogen) sensitive type baicalin
The technology of body gel and baicalin is applied in the preparation of the gel preparation, and the pH-sensitive baicalin eye is used in the field of body gel preparation, which can solve the problems of low bioavailability, loss of baicalin and the like, and achieves the composition of Reasonable, solve the effect of excessive metabolism and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
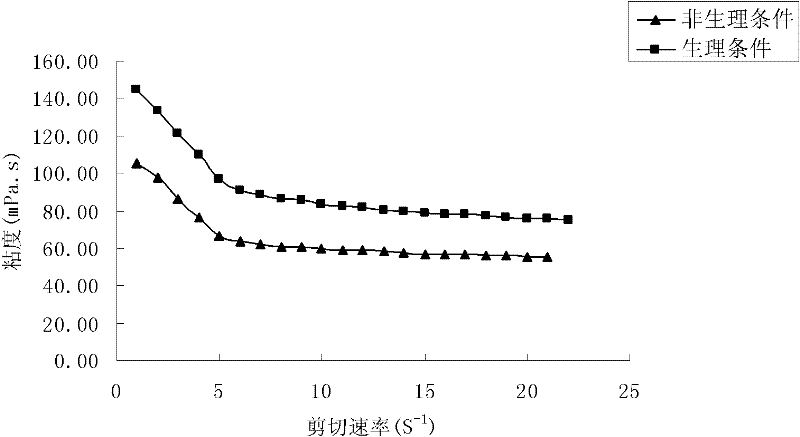
Image
Examples
Embodiment 1
[0048] Preparation of Example 1pH Sensitive Baicalin Ophthalmic In-body Gel (1)
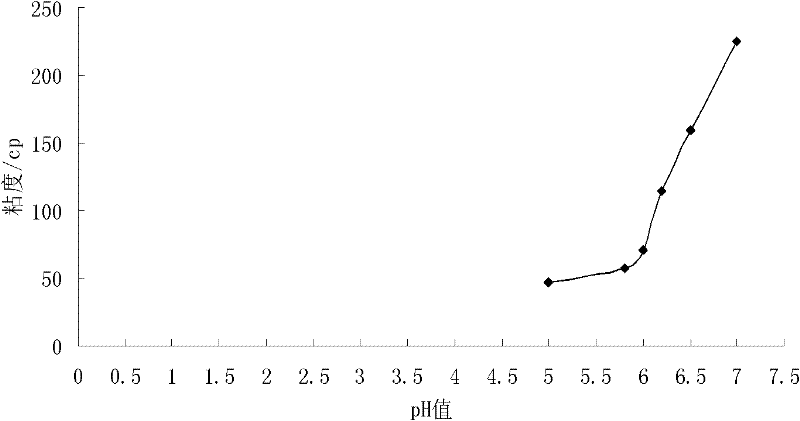
[0049] The composition of the drug prescription is: 0.1 part of baicalin, 0.3 part of carbomer 974P NF 974P NF), 0.6 parts of HPMC E4M, 0.9 parts of sodium chloride, 0.001 parts of benzalkonium bromide, and 94 parts of phosphate buffered saline (PBS) at pH 5.8.
[0050] The preparation method of the above-mentioned pH-sensitive ophthalmic in-body gel preparation comprises the following steps:
[0051] (1) preparation matrix: get the described ratio Add an appropriate amount of pH5.8 phosphate buffer with HPMC E4M under continuous stirring, and stir evenly to obtain the matrix;
[0052] (2) Prepare the drug solution: take the baicalin, sodium chloride and benzalkonium bromide in the ratio, gradually add them to the phosphate buffer solution with pH 5.8, and mix evenly to obtain the drug solution;
[0053] (3) Mixing: Add the drug solution prepared in step (2) into the matrix obtained in step ...
Embodiment 2
[0054] Preparation of embodiment 2pH-sensitive baicalin ophthalmic in-body gel (2)
[0055] The composition of the drug prescription is: 0.1 part of baicalin, 0.3 part 980NF, 0.6 parts of HPMC F4M, 0.9 parts of sodium chloride, 0.001 parts of benzalkonium bromide, and 94 parts of phosphate buffer at pH 5.8.
[0056] The preparation method of the pH-sensitive ophthalmic in-body gel preparation is the same as that in Example 1.
Embodiment 3
[0057] Preparation of Example 3 pH Sensitive Baicalin Ophthalmic In-body Gel (3)
[0058] The composition of the drug prescription is: 0.1 part of baicalin, 0.3 part 934P NF, 0.6 parts of HPMC K4M, 0.9 parts of sodium chloride, 0.001 parts of benzalkonium bromide, and 94 parts of phosphate buffer at pH 5.8.
[0059] The preparation method of the pH-sensitive ophthalmic in-body gel preparation is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com