Medicinal composition and preparation method thereof
A composition and drug technology, applied in drug combinations, pharmaceutical formulations, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1 golden buckwheat extraction process
[0103] Pulverize the golden buckwheat crude drug and pass through a 50-mesh sieve to obtain golden buckwheat coarse powder; add 2kg of golden buckwheat coarse powder into 50% ethanol solution of 10 times the amount (weight ratio, the same below), heat and reflux twice at 60°C , 1.5 hours each time, combine the filtrates, filter, and concentrate the filtrate under reduced pressure at 60°C to no alcohol smell, add an appropriate amount of 2.1kg of water to dissolve, filter with suction, and set aside. Take the pretreated D-101 macroporous resin and pack it into a column. The flow rate of the drug solution is 2 resin column volumes / hour, and the adsorption is for 4 hours. First, 6 column volumes of water are used to elute and remove impurities. The flow rate of impurity removal is 4 resin columns. volume / hour, then eluted 8 column volumes with 70% ethanol, and the elution flow rate was 2 resin column volumes / hour, reclaime...
Embodiment 2
[0104] Example 2 Extraction process of Evodia rutaecarpa
[0105]Pulverize the crude drug of Evodia rutaecarpa, pass through a 50-mesh sieve to obtain coarse powder of Evodia rutaecarpa; add 10 times the amount of hydrochloric acid ethanol (alcohol concentration is 80%) solution with a pH value of 3 to 6kg of Evodia rutaecarpa coarse powder, heat and reflux 3 times, each time 1.5 h, filter, combine the filtrates, concentrate to 50°C and measure the relative density to be 1.20, let cool to obtain a concentrated solution, adjust the pH value of the concentrated solution to 2, let stand, and centrifuge to obtain 4000 ml of a supernatant, which is set aside. Take 4000ml of 732 strong-acid cation exchange resin, pack it into a column, the diameter-to-height ratio is about 1:10, and the column adsorption flow rate of the liquid medicine is 1 resin column volume / hour. Until the effluent is colorless, the elution flow rate is 2 resin column volumes / hour; the 80% ethanol eluate is coll...
Embodiment 3
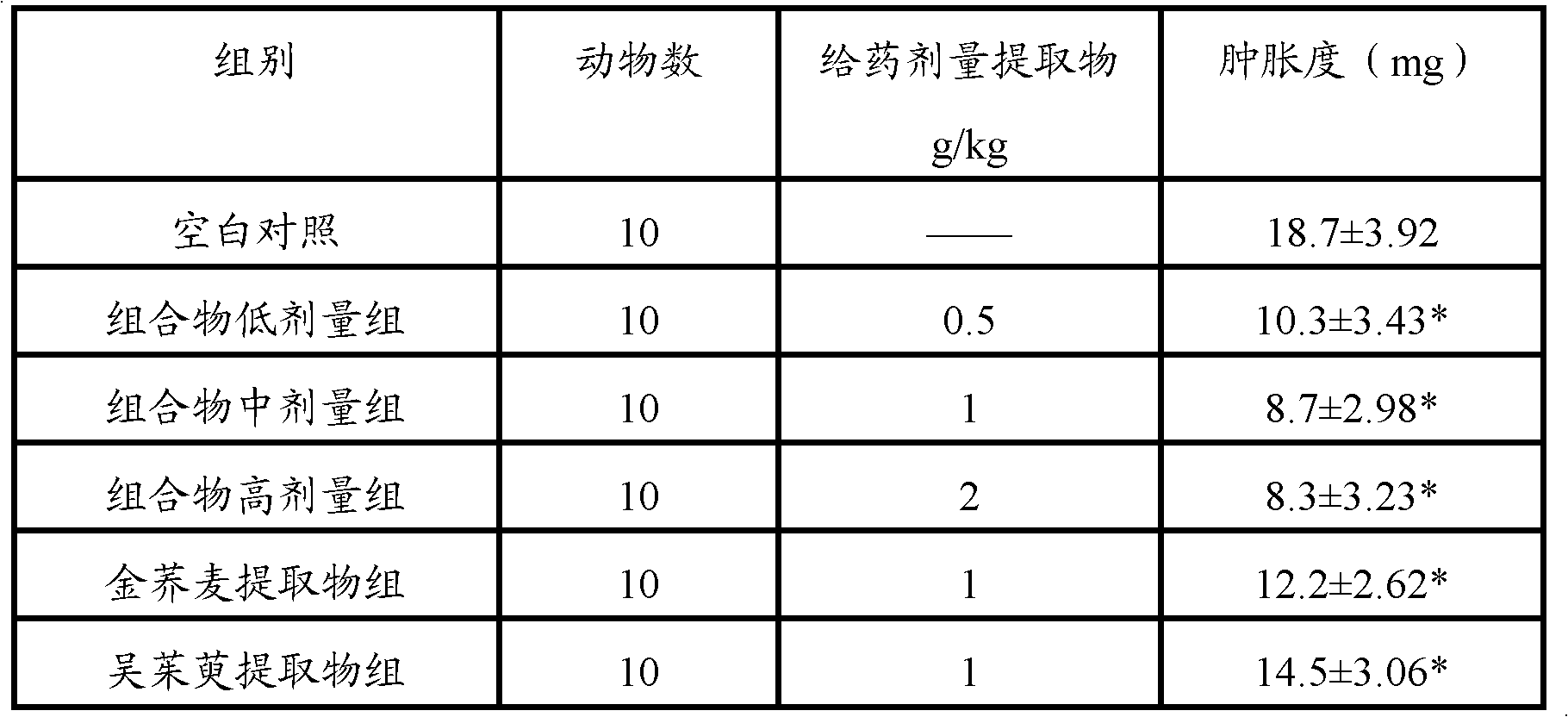
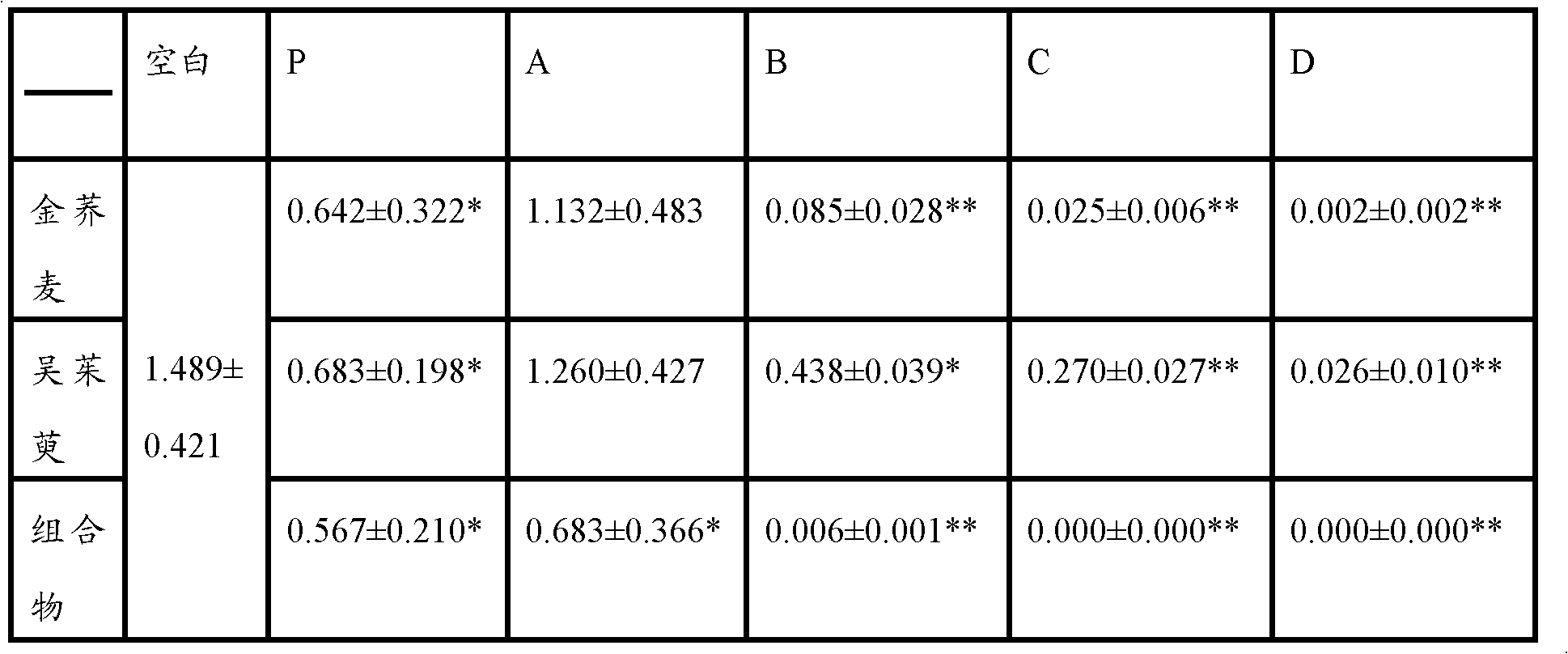
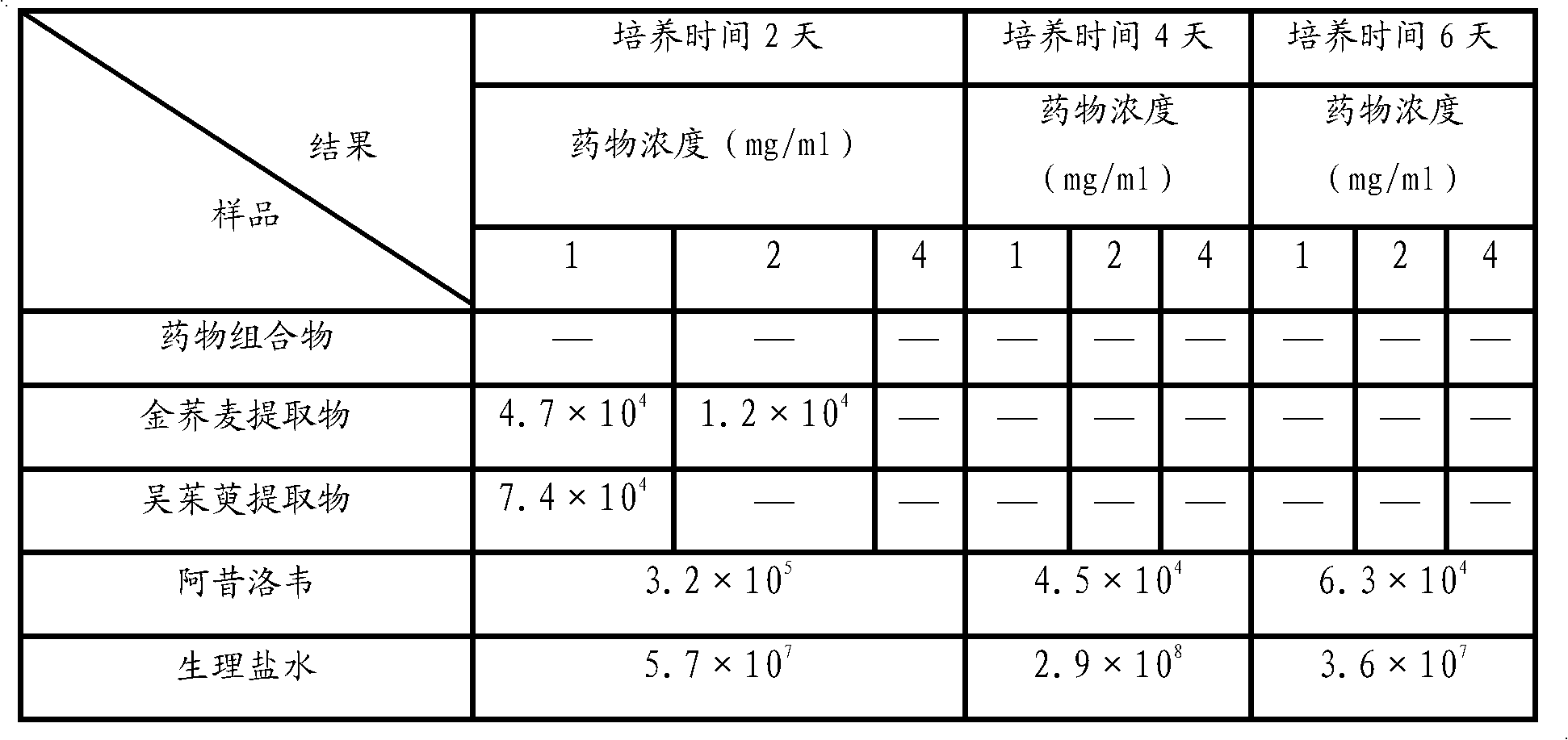
[0106] Embodiment 3 compares the anti-inflammatory effect when the pharmaceutical composition of the present invention and two kinds of pharmaceutical components are used alone
[0107] 1. Animals and materials
[0108] (1) Animals
[0109] 1. Species: Kunming mice, provided by the Experimental Animal Center of Wuhan University School of Medicine, animal certificate number: 19-013;
[0110] 2. Weight: 18-22g;
[0111] 3. Gender: half male and half male;
[0112] 4. Age: adult and healthy;
[0113] 5. Number of animals: 60.
[0114] (2) Test substance
[0115] 1. Tested substance: golden buckwheat extract (prepared according to Example 1), Evodia rutaecarpa extract (prepared according to Example 2), golden buckwheat extract (prepared according to Example 1) and Evodia rutaecarpa extract (according to Example 2 Preparation) uniformly mix the prepared pharmaceutical composition at a weight ratio of 1:1; aspirin is used as the positive control drug.
[0116] 2. Content: The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com