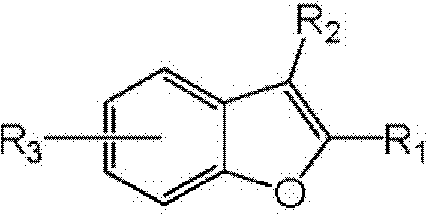
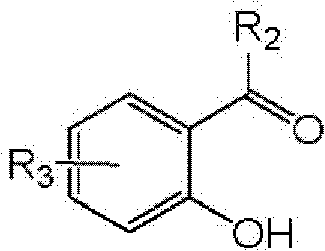
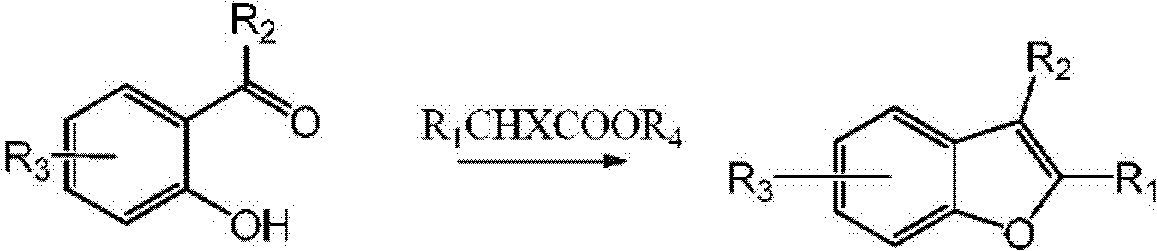Preparation process of benzofuran
A technology for benzofuran and preparation process, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low ring-closing yield, difficulty in scale-up production, and high operating costs, and achieves the effects of short reaction time, easy purification, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1.2, the preparation of 3-dimethyl-6-methoxybenzofuran
[0044]
[0045] Add DMSO (80ml), 2-hydroxyl-4-methoxyacetophenone (5g, 0.03mol) in 250ml reaction bottle, add Cs at room temperature 2 CO 3 (25g, 0.077mol), and 2-chloropropionic acid (3.5g, 0.032mol) was added dropwise under stirring, and the dropping temperature was controlled below 60°C. After the dropwise addition, the temperature was raised to 100-120°C to react for 2 hours, the reaction was quenched by adding water, the pH was adjusted to 1 with hydrochloric acid, the product was extracted with organic solvent, washed with water, and purified by short column chromatography to obtain 4.9 g of the product with a yield of 92%. HNMR (ppm, CDCl 3 ): 7.33-7.35(d, 1H), 6.98-700(d, 1H), 6.83-6.85(d, 1H), 3.82(s, 3H), 2.11(s, 3H), 2.32(s, 3H).
Embodiment 2
[0046] The preparation of embodiment 2.2-methyl-6-methoxybenzofuran
[0047]
[0048] Add DMF (50ml) and 2-hydroxy-4-methoxybenzaldehyde (5g, 0.032mol) to a 250ml reaction flask, add NaH (2.4g, 0.1mol) at room temperature, and add 2-bromopropionic acid ethyl Ester (6g, 0.033mol), heated to 100-120°C for 2 hours, quenched with water, adjusted to pH=1 with hydrochloric acid, extracted with organic solvent, washed with water, and purified by column chromatography to obtain 4.95g of product with a yield of 95%. HNMR (ppm, CDCl 3 ): 7.28-7.30 (d, 1H), 6.94-6.95 (d, 1H), 6.78-6.81 (dd, 1H), 6.25 (s, 1H), 3.80 (s, 3H), 2.39 (s, 3H).
Embodiment 3
[0049] The preparation of embodiment 3.2-ethyl-3-methylbenzofuran
[0050]
[0051]Add NMP (80ml), 2-hydroxyacetophenone (5g, 0.037mol) in the 250ml reaction bottle, add K at room temperature 2 CO 3 (15g, 0.11mol), added dropwise ethyl 2-bromobutyrate (7.25g, 0.037mol) under stirring, raised the temperature to 100-120°C for 2 hours, quenched the reaction with water, adjusted the pH to 1 with hydrochloric acid, extracted, Washing with water and purification by column chromatography yielded 5.12 g, with a yield of 86%. HNMR (ppm, CDCl 3 ); 7.44-7.46 (m, 2H), 7.23-7.25 (m, 2H), 2.65 (q, 2H), 2.05 (s, 3H), 1.21 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com