Method for preparing sulfoacid/carboxylic acid waterborne polyurethane
A technology of waterborne polyurethane and carboxylic acid type, which is applied in the field of preparation of sulfonic acid/carboxylic acid type waterborne polyurethane, can solve the problems of single type and performance, and achieve the effects of high product yield, simple process route, and regularity protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 2.5mol of toluene diisocyanate (435g) and 1mol of polycaprolactone diol (2000g) with a molecular weight of 2000 to a reactor equipped with a stirrer, reflux condenser, nitrogen protection, and temperature indication, and add 1.2g of catalyst Bicat8108 (20%Bi) was reacted at 80°C for 1.5h to obtain product A;
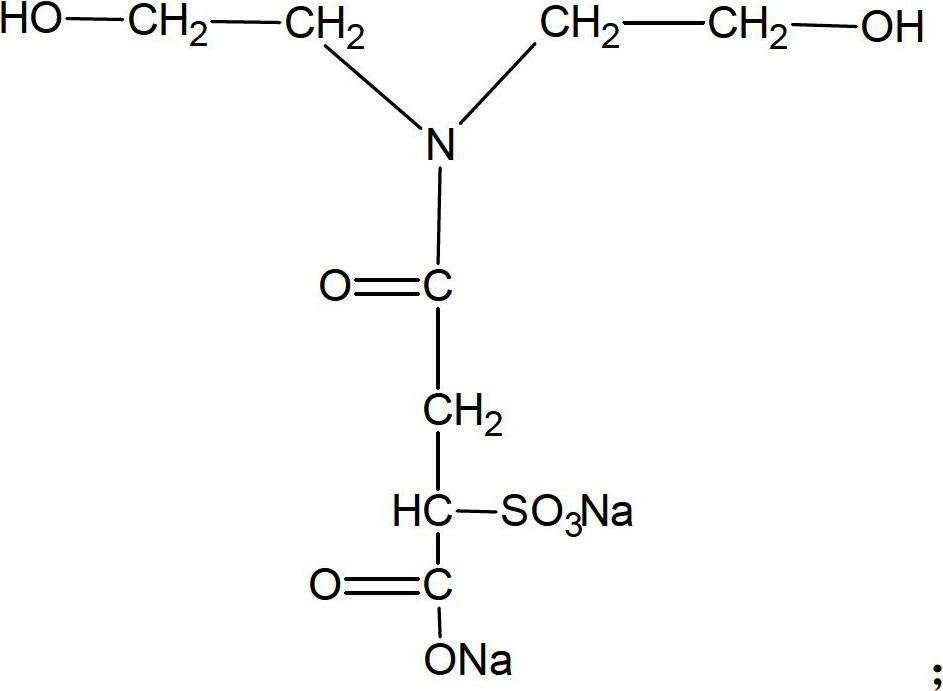
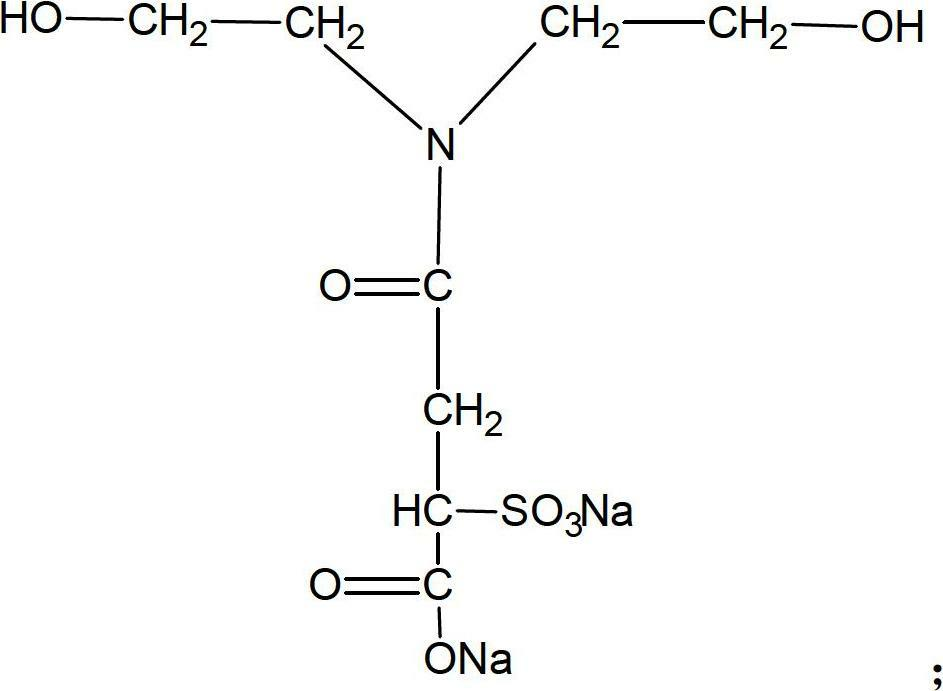
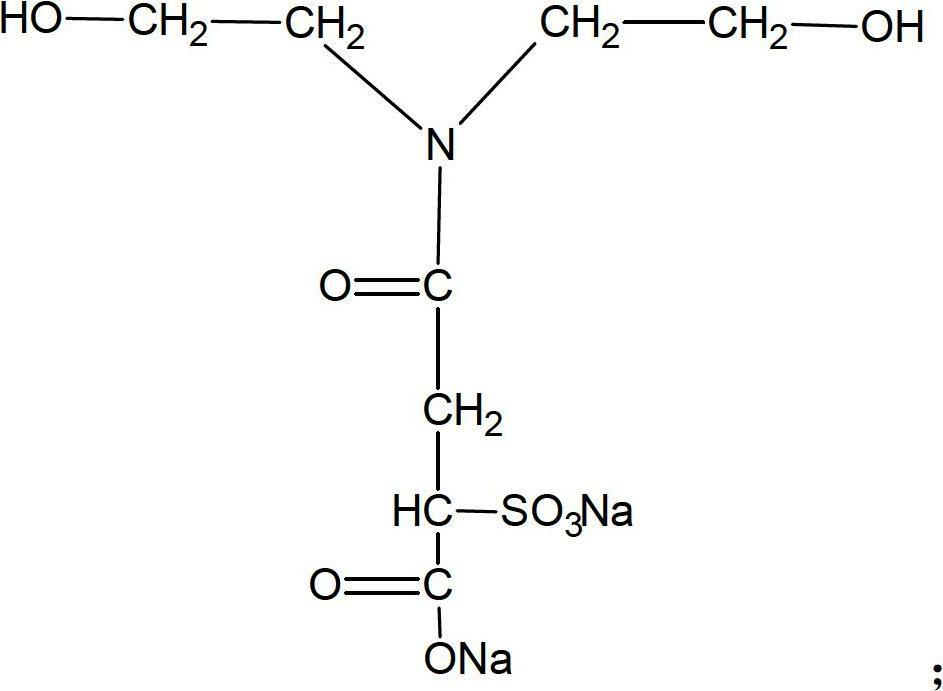
[0027] (2) Add 36.5g of sulfonic acid / carboxylic acid type hydrophilic chain extender and 6.2g of ethylene glycol chain extender into the reactor, add an appropriate amount of acetone into the reactor according to the viscosity (viscosity control is not greater than 20000CPS), React at 70°C for 3 hours to obtain product B (waterborne polyurethane hydrophilic prepolymer). The molecular structure of the sulfonic acid / carboxylic acid type hydrophilic chain extender is as follows:
[0028]
[0029] (3) Cool the product B (hydrophilic prepolymer of water-based polyurethane) to below 50°C, add it into 2500g of water for dispersion under high-speed stirring, a...
Embodiment 2
[0032] (1) Add 2mol of 4,4-diphenylmethane diisocyanate (500g) and 1mol of polytetrahydrofuran diol (2000g) with a molecular weight of 2000 into a reactor equipped with a stirrer, reflux condenser, nitrogen protection, and temperature indication , adding 1.25g of catalyst and reacting at 85°C for 1h to obtain product A;
[0033] (2) Add 75g of sulfonic acid / carboxylic acid type hydrophilic chain extender and 9g of 1,4-butanediol chain extender to the reactor, and add an appropriate amount of acetone into the reactor according to the viscosity (viscosity control is not greater than 20000CPS), React at 75°C for 2 hours to obtain product B (waterborne polyurethane hydrophilic prepolymer). The molecular structure of the sulfonic acid / carboxylic acid type hydrophilic chain extender is as follows:
[0034]
[0035] (3) Cool the product B (hydrophilic prepolymer of waterborne polyurethane) to below 50°C, add it to 3800g of water for dispersion under high-speed stirring, add 3.8g o...
Embodiment 3
[0038] (1) Add 3mol of 1,6-hexyl diisocyanate (505g) and 1mol of polyoxypropylene diol (1000g) with a molecular weight of 1000 into a reactor equipped with a stirrer, reflux condenser, nitrogen protection, and temperature indication , add 0.75g of catalyst, react at 90°C for 2h, and obtain product A;
[0039] (2) Add 60g of sulfonic acid / carboxylic acid type hydrophilic chain extender, 25g of ethylene glycol, and 36g of 1,4-butanediol into the reactor, and add an appropriate amount of butanone according to the viscosity (viscosity control is not greater than 20000CPS) Put into the reactor and react at 85°C for 4 hours to obtain product B (waterborne polyurethane hydrophilic prepolymer). The molecular structure of the sulfonic acid / carboxylic acid type hydrophilic chain extender is as follows:
[0040]
[0041] (3) Cool the product B (hydrophilic prepolymer of waterborne polyurethane) to below 50°C, add it into 1100g of water to disperse under high-speed stirring, add 2g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com