Method for preparing toluene diamino butyl formate
A technology of butyl toluene dicarbamate and toluene diamine, which is applied in the field of preparation of butyl toluene dicarbamate, can solve the problems of difficult separation of catalysts, easy deactivation of catalysts, and impact on production costs, so as to avoid adverse effects and reuse Good performance and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
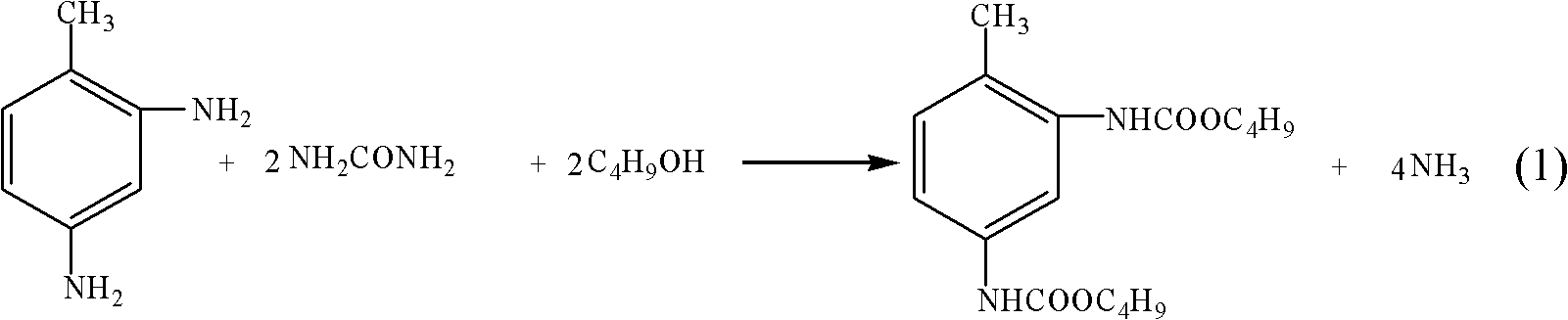
[0022] Add 0.095mol 2,6-toluenediamine, 0.19mol urea, 0.95mol n-butanol, and 0.580g Hβ molecular sieve roasted at 300°C to the reactor in sequence, then seal the reactor, and heat it to the temperature of The reaction was carried out at 130°C for 10 hours. After the reaction, the temperature was lowered, the material was discharged, and the catalyst was separated by filtration at 30°C. The composition of the filtrate was analyzed by high performance liquid chromatography, and the yield of the product butyl toluene dicarbamate was 3.5%.
Embodiment 2
[0024] In the reactor, add 0.02mol molar ratio of 2,4-diaminotoluene: 2,6-diaminotoluene=80:20 mixture, 0.08mol urea, 1.4mol n-butanol, 0.732g roasted at 400°C HY molecular sieve, then seal the reaction kettle, under the condition of stirring, heat to 170°C to react, react for 1 hour, after the reaction, cool down, discharge, filter and separate the catalyst at 50°C, and analyze the filtrate with high performance liquid chromatography Composition, the measured yield of product butyl toluene dicarbamate is 25.0%.
Embodiment 3
[0026] 0.03mol molar ratio of 2,4-diaminotoluene: 2,6-diaminotoluene=65:35 mixture, 0.18mol urea, 1.5mol n-butanol, 1.098g roasted γ-Al 2 o 3 Catalyst, then seal the reaction kettle, under stirring condition, be heated to the temperature and react under 190 ℃, react for 6 hours, cool down after the reaction finishes, discharge, and filter and separate the catalyzer at 80 ℃, analyze the filtrate composition with high performance liquid chromatography, The yield of the product butyl toluene dicarbamate was measured to be 62.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com