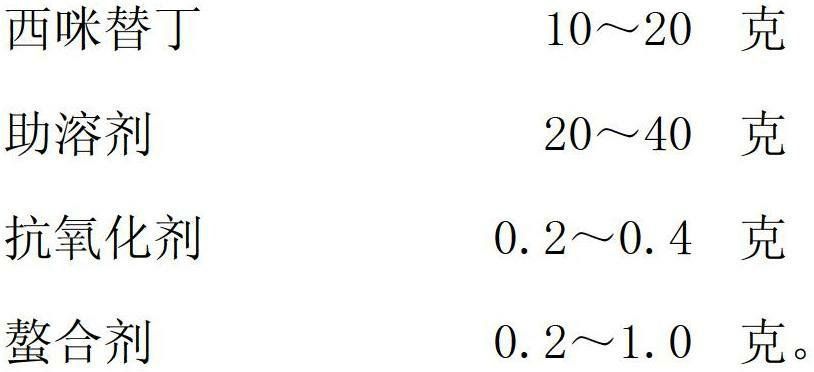Cimetidine injection and preparation process
A technology of cimetidine and injection, which is applied in the field of preparation of cimetidine medicaments, can solve the problems of animal body stress response, unfavorable disease treatment, poor palatability, etc., and achieve reduced injection times, high active ingredient content, The effect of avoiding harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of above-mentioned cimetidine injection comprises the following steps:
[0023] (1) Mix cimetidine with water for injection to form a suspension;
[0024] ⑵ After adding co-solvent to the suspension, continue to add water for injection to make the suspension into a colorless and transparent solution;
[0025] ⑶ adding antioxidants;
[0026] (4) adding chelating agent;
[0027] (5) Dilute the volume to 100 ml with water for injection, filter and sterilize to obtain the finished product. Advantage and positive effect of the present invention are:
Embodiment 1
[0029] (1) Mix 10 grams of cimetidine with 50 ml of water for injection to form a suspension;
[0030] ⑵ Add 20 grams of tartaric acid to the suspension, and then continue to add water for injection to make the suspension into a colorless and transparent solution;
[0031] (3) Add 0.2 gram of sodium metabisulfite;
[0032] (4) Add 0.2 grams of EDTA;
[0033] (5) Dilute the volume of water for injection to 100ml, filter and sterilize with a 0.22-micron filter membrane, and make a finished product after inspection and filling.
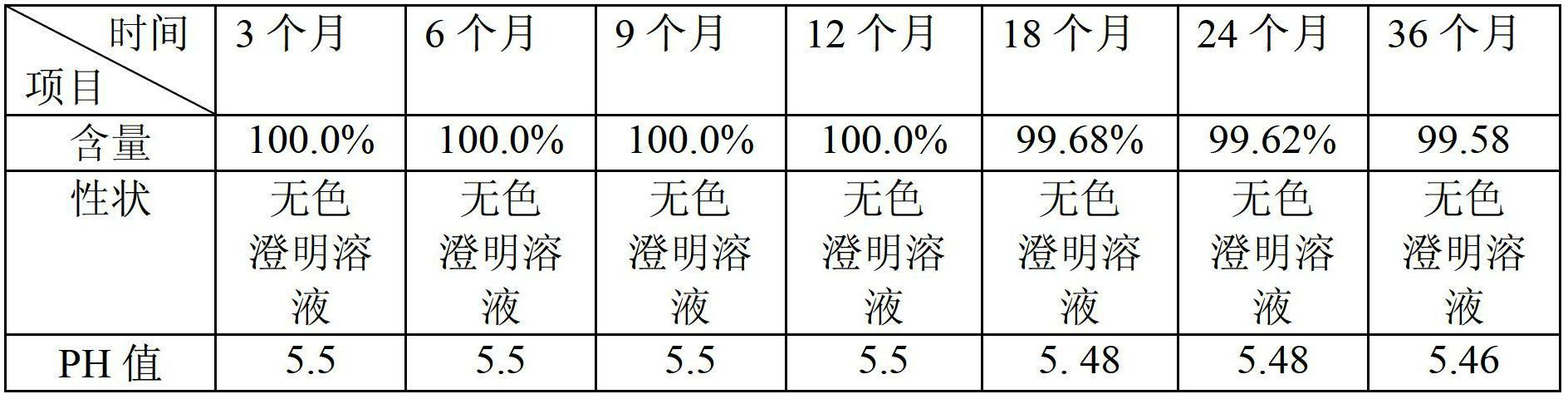
[0034] The active ingredient content of the prepared cimetidine injection is: 10%.
Embodiment 2
[0036] (1) Mix 15 grams of cimetidine with 50 ml of water for injection to form a suspension;
[0037] ⑵ Add 10 grams of lactic acid and 15 grams of butyrosine to the suspension, and then continue to add water for injection, so that the suspension forms a colorless and transparent solution;
[0038] (3) Add 0.4 gram of sodium thiosulfate;
[0039] (4) Add 1.0 g of EDTA;
[0040] (5) Dilute the volume of water for injection to 100ml, filter and sterilize with a 0.22 micron filter membrane, and make the finished product after inspection and filling.
[0041] The active ingredient content of the prepared cimetidine injection is: 15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com